Planning and delivering radiotherapy to moving targets is an enduring challenge. Tumors can change shape, location, and functionality between treatment fractions, while patients may undergo anatomical changes during a radiotherapy course. Tumors and organs-at-risk can also move during treatment delivery -- and not necessarily in sync with each other. For proton therapy, in which motion can introduce density changes in the beam path, the implications of such uncertainties can be particularly severe.
This "4D radiotherapy problem" is not a recent realization; attempts at 4D treatment planning to account for motion and deformation have been ongoing for at least two decades. But while recent years have seen many interesting technology developments, clinical transfer of these techniques is not easy.
 Around 60 attendees from around the world attended the 4D Treatment Planning Workshop, an annual event held this year in the U.K. Image courtesy of Antje-Christin Knopf.
Around 60 attendees from around the world attended the 4D Treatment Planning Workshop, an annual event held this year in the U.K. Image courtesy of Antje-Christin Knopf."We are making incremental progress," said Uwe Oelfke, PhD, head of the Joint Department of Physics at the U.K. Institute of Cancer Research and the Royal Marsden in London. "But a big breakthrough pushing into the clinic has not been achieved."
Oelfke was speaking at the opening session of the annual 4D Treatment Planning Workshop, held last month in the U.K. The workshop, which featured talks by world-leading experts, saw around 60 attendees come together to discuss all aspects of planning and delivering photon and particle therapy to moving targets.
One area examined in detail was the use of imaging for monitoring motion during treatment and reoptimizing the plan accordingly. In particular, the speakers examined how to do this without the use of ionizing radiation. "X-rays are not the future," Oelfke said. "I believe in MR and ultrasound for real-time imaging of anatomy."
MR motion monitoring
 Bas Raaymakers, from the University Medical Center Utrecht in the Netherlands, is part of the MR Linac Research Consortium that's working to move MR-linac technology into the clinic.
Bas Raaymakers, from the University Medical Center Utrecht in the Netherlands, is part of the MR Linac Research Consortium that's working to move MR-linac technology into the clinic.Bas Raaymakers, PhD, from the University Medical Center Utrecht (UMCU) in the Netherlands discussed the use of MR guidance for 4D treatment planning. The Utrecht team is heading up the development of an integrated MR-linac for MR-guided radiotherapy. The system, which is currently undergoing clinical installation at UMCU, comprises a 6 MV accelerator rotating on a ring gantry around a closed-bore 1.5-tesla MR scanner.
"The goal is to use MR images as feedback for adapting treatment while the patient is on the treatment table," he said. He noted that as well as offering exquisite soft-tissue contrast, MRI is incredibly versatile and can image a wide variety of structures. The ability to visualize lymph nodes, for example, enables the use of stereotactic boost to individual nodes. "This really opens up new possibilities for radiotherapy," he said.
MRI also offers the ability to perform true 4D imaging -- repeated acquisition of 3D image volumes. Raaymakers showed an example 4D MRI of the pelvis recorded at UMCU using a 3D T1-FFE sequence. Images with a spatial resolution of 3 x 3 x 6 mm were acquired with a temporal resolution of 2-3 seconds. Thorax MRI using the same approach achieved a temporal resolution of 3 seconds, though this anatomy showed more image artifacts.
Importantly, he pointed out, MR image quality is not affected by the accelerator, with imaging quality maintained whether the treatment beam is on or off. This feature enables continuous high-speed imaging during beam delivery; then if changes are seen in tumor or patient anatomy, the treatment plan can be adapted accordingly.
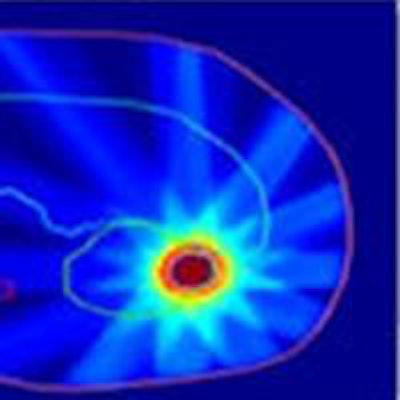
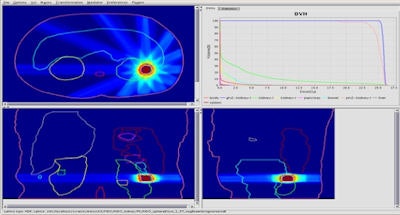 Kidney treatment plan created using the team's fast IMRT optimization system. Image courtesy of Bas Raaymakers.
Kidney treatment plan created using the team's fast IMRT optimization system. Image courtesy of Bas Raaymakers.To do this, the UMCU team developed a fast intensity-modulated radiotherapy (IMRT) optimization system that combines a GPU-based Monte Carlo dose calculation engine for beamlet generation with a fast inverse dose optimization algorithm. The resulting algorithm is fast enough to perform daily reoptimization.
Whilst conventional radiotherapy systems use couch motion to account for changes in tumor or patient location, the MR-linac uses "virtual couch shift" (VCS). Here, all adjustments are performed within the treatment-planning environment. The dose distribution is kept the same, maintaining the pretreatment patient specific considerations, but is translated and/or rotated to adapt to the patient's daily geometry.
In a move toward intrafraction reoptimization, Raaymakers presented the adaptive sequencer (ASEQ) algorithm. ASEQ comprises four steps: dose optimization (with the first dose used as the optimal dose); segmentation; calculation of the dose delivered by one segment; and subtraction of this segment dose from the target dose. The four-step process is performed for one segment per cycle, and then repeated to incorporate all of the segments.
He also described a study using MRI-based dose reconstruction for gated delivery. Here, the position of a moving phantom was tracked with the MR scanner and gated radiation delivery performed based on this position. Dose distributions with gating were similar to a static case, while nongated distributions were highly distorted. This use of position data for on-line reconstruction of accumulated dose could potentially be used for intrafraction plan adaptation.
Raaymakers concluded by looking at the introduction of MR-guided radiotherapy into the clinic. In one aspect, MRI can improve existing radiotherapy methods by examining the daily state of the patient and creating a new plan each day. MR guidance can also open up opportunities to treat new patient categories, such as those with small renal cell tumors.
"MRI can see these tumors, can enable gating and tracking," he said. "Overall, we're using MRI as a feedback loop to guide what we're doing."
Ultrasound options
Another approach for nonionizing, noninvasive imaging of patient and tumor motion is to use ultrasound guidance. "There are many ways to implement ultrasound into the radiation treatment chain," Emma Harris, PhD, from the Institute of Cancer Research (ICR), told the workshop attendees.
Ultrasound offers many advantages over existing approaches for radiotherapy motion management, including: superior soft-tissue imaging to x-rays; zero dose and low cost; no need for fiducials; high temporal resolution; and compatibility with particle therapy. Real-time ultrasound imaging in 3D is now also possible, enabling 4D monitoring of target positions before or during treatment.
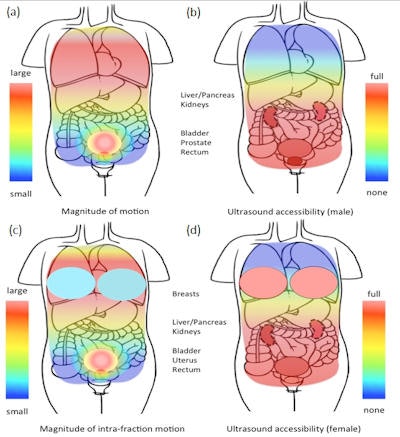 Schematic representation of the varying magnitude of tissue intrafraction motion (a) and (c) and the varying accessibility of tissue by ultrasound for the purpose of intrafraction motion tracking (b) and (d), in the male and female upper torso. Image courtesy of Emma Harris.
Schematic representation of the varying magnitude of tissue intrafraction motion (a) and (c) and the varying accessibility of tissue by ultrasound for the purpose of intrafraction motion tracking (b) and (d), in the male and female upper torso. Image courtesy of Emma Harris.There are, however, challenges to consider. Firstly, ultrasound waves don't propagate easily through air or bone, so anatomy such as ribs and lung should be avoided. This restricts the useful maximum depth for accurate ultrasound motion estimation to approximately 20 cm, limiting its use in very large patients. Reproducible probe pressure is required to minimize variations in tissue displacement. In addition, many radiotherapists are unfamiliar with using ultrasound.
Creating 3D images also takes time. With a swept probe approach, in which a conventional linear-array transducer is moved mechanically to create a 3D image, acquisition speed is limited to one to two volumes per second. A 2D matrix array transducer can create one to two thousand volumes per second. However, this newer technology is not yet commonly applied within radiotherapy.
Currently, there's only one commercial system available for 4D ultrasound guidance during radiotherapy -- radiation oncology firm Elekta's Clarity Autoscan system for prostate cancer. "Clarity is being used widely for interfraction imaging and some initial intrafraction imaging studies have been reported. However, it has not been used to gate or track treatments as yet," Harris said. "But it is ready to go for this application." She described several studies using Clarity to track moving phantoms with tracking errors of around 1 mm.
In vivo, submillimeter tracking has been demonstrated in the prostate and liver. Harris cited a study from Stanford University using transabdominal ultrasound to image five volunteers, with a robotic arm employed to control probe pressure and position. "The researchers found that they could gate treatment reliably before the prostate moved more than 3 mm or 5 degrees," she said.
Harris and colleagues performed an in vivo study using 4D ultrasound to track displacement of blood vessels in the liver. Ultrasound- and manually-tracked displacements agreed to within 1.7 mm. In another study, they used a 2D matrix array probe to increase acquisition speeds, revealing that a frame rate of 8-12 Hz was needed for a tracking accuracy of below 1 mm.
Looking toward intrafraction ultrasound guidance, it's also important to assess the impact of the probe on the radiation beam. Harris described a study at John Hopkins University in which stereotactic body radiotherapy plans were created for 10 liver cancer patients, with and without the probe in place. In eight patients, no significant difference was seen between the two plans. In the other two, however, the tumor position restricted the treatment quality when the probe was present. She reported that the same group is also developing a cooperative robotic arm, which can be used to reduce probe pressure variability and help radiotherapists with probe placement.
Intrafraction tracking
Continuing the theme of intrafraction guidance, Harris' colleague Tuathan O'Shea rounded off her presentation with a closer look at ultrasound's potential for tracking intrafraction motion during radiotherapy. In particular, he examined whether 4D speckle tracking can accurately track prostate displacement.
 Emma Harris and Tuathan O'Shea, from the Institute of Cancer Research, discussed ultrasound guidance for radiotherapy.
Emma Harris and Tuathan O'Shea, from the Institute of Cancer Research, discussed ultrasound guidance for radiotherapy.O'Shea described a recent study at the International Congress of Radiology (ICR) comparing motion tracking using a 3D swept linear-array ultrasound probe with the CyberKnife's fiducial-based x-ray tracking system. The researchers imaged a phantom moving according to five patient motion traces. Motion was detected directly from the ultrasound images by comparing the speckle pattern in a reference region in one image frame with patterns in subsequent frames, with 98.4% of estimates within ± 0.5 mm of the input traces.
"Eventually, full 3D high-volume-rate motion estimation will be possible," he said. "However for the moment, since the right-left motion is so small for the prostate, we are evaluating with current imaging technology whether low-volume-rate full-3D tracking offers any advantages over high-frame-rate 2D tracking."
They also studied ultrasound-based gating, showing that 3D ultrasound images could accurately detect displacement thresholds of 2.0 mm and 5.0 mm in the axial and lateral directions, with mean errors of less than 0.4 mm. O'Shea noted that left-right prostate movement is very small, citing a study of 22 patients in which only one of 480 fractions exhibited motion greater than 2 mm. "If the right-left motion is so small for the prostate, do you really need to track it in 3D?" he asked, pointing out that a return to 2D ultrasound imaging would allow higher-frame-rate imaging.
Another question is whether ultrasound motion estimation remains accurate during long sequences relevant for radiotherapy. O'Shea described the recent MICCAI challenge, in which ultrasound imaging was used to track blood vessels in the liver during free breathing, over long sequences of up to 9.68 minutes.
He explained that problems arose if a similar looking object appeared within the search region. To improve motion estimation, he suggested a temporal regularization method, in which the initial motion estimation is corrected using a-priori knowledge of motion behavior. "A temporal regularization scheme based on error detection and motion prediction can improve motion estimation in long ultrasound sequences," O'Shea concluded.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.