The sustained clinical progress being made in the use of MRI to evaluate cardiac function in sickle cell disease (SCD) and identify inflammation as a cause of graft dysfunction in heart transplant patients was evident in two separate eye-catching presentations made at this week's European Society of Cardiology (ESC) congress.
SCD affects about 15,000 people in the U.K. and can lead to progressive end organ damage with cardiac dysfunction, resulting in significant morbidity and mortality, noted Dr. Jessica Webb, consultant cardiologist at Guy's and St. Thomas' Hospital NHS Foundation Trust in London, first author Monica Bawor, PhD, and colleagues in an e-poster presentation at the virtual congress, which took place from 29 August to 1 September.
Heart failure (HF) in SCD remains a clinical challenge due to the complexity of cardiac involvement. Pulmonary hypertension, left ventricular diastolic dysfunction, atrial enlargement, and valvular disease are some of the ways in which HF is manifested in this population.
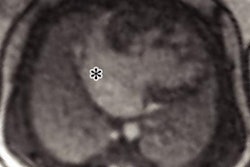
"The cardiac abnormalities associated with heart failure in SCD have rarely been investigated with cardiac MRI," the authors stated, adding that cardiac MRI is the gold-standard modality for evaluating cardiac function and therefore can provide valuable information in the assessment of HF in patients with SCD.
The ESC updated its definition of HF in 2016 to include three categories: HF with reduced ejection fraction (HFrEF), also known as systolic HF; HF with midrange ejection fraction (HFmrEF); and HF with preserved ejection fraction (HFpEF). The prevalence of HF in SCD under this updated classification is not well known, they explained.
The group reviewed all patients with SCD at Guy's and St. Thomas' who had been referred for cardiac MRI between 2011 and 2019. Cardiac MRI was performed in patients with abnormal screening echo or unexplained cardiorespiratory symptoms or for monitoring of iron overload.
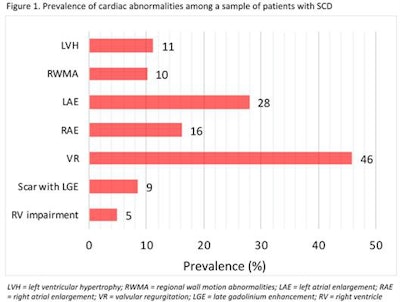
The researchers collected data on measures of cardiac function, including left and right ventricular fractions, ventricular hypertrophy, atrial enlargement, regional wall motion abnormalities, valvular disease, myocardial scarring, and cardiac iron load.
The total sample size was 82 (68% female, 32% male), and the average at the time of scan was 37 years. The authors reviewed 123 scans. The average left ventricular fraction was 57%, and the prevalence of cardiac abnormality was 60%. A total of 23 patients (28%) met the criteria for HF. The prevalence of HFrEF was 10% (8 patients), the prevalence of HFmrEF was 7% (six patients), and the prevalence of HFpEF was 11% (nine patients).
SCD affected cardiac function in over half of patients referred for cardiac MRI, Webb and colleagues concluded. "This study has implications for the diagnosis and subsequent management of cardiac abnormalities in this population and it can be used to further investigate and guide the development of targeted treatments for these patients," they wrote.
Heart transplants at Mayo Clinic
In another ESC presentation, U.S. researchers showed that cardiac MRI can identify inflammation as a potential cause of graft dysfunction (GD) in around half of heart transplant patients. Heart transplant patients can develop GD without biopsy evidence of cell or antibody mediated rejection, and cardiac MRI can detect inflammatory or infiltrative causes of cardiomyopathy, explained Dr. Senthil Anand, cardiology fellow at Mayo Clinic Arizona, and colleagues in an e-poster.
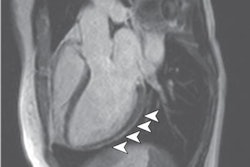
"There are two distinct patterns of late gadolinium enhancement (LGE) observed in graft dysfunction: diffuse epicardial (56%) and patchy (44%)," they wrote. "Although the presence of LGE in itself is not associated with myocardial recovery, 90% of patients with a diffuse epicardial pattern have recovery of graft dysfunction."
In their study, 37 heart transplant patients (34% women, mean age at transplant = 49 ± 13 years) presented with acute decline in left ventricular ejection fraction (LVEF) of less than 50% and more than 10% from the baseline, with no biopsy evidence of rejection between 2007 and 2018. Twenty-five patients presented with heart failure, and one presented with cardiac shock. The other 11 were asymptomatic.
A coronary angiogram with intravascular ultrasound was performed to rule out allograft vasculopathy. Responders were defined as those with LVEF improvement of more than 10% at 180 days or greater postpresentation. Cardiac MRI determined left and right ventricular indices and pattern of LGE.
Most patients were treated with steroid bolus (n = 29) and/or plasmapheresis (n = 23). The researchers found no major changes made in immunosuppression in six patients. Delayed enhancement MRI was performed in with standard inversion-recovery gradient echo sequences, between 5 and 20 minutes after standard protocol administration of intravenous gadolinium contrast.
Biventricular LGE was present in 18 out of 37 (49%) patients with GD, and was more prevalent in responders (57%, 13 out of 23) than nonresponders (35%, 5 out of 14), although not statistically significant (p = 0.21).
A diffuse epicardial pattern of late gadolinium enhancement was identified in 10 patients, out of which nine were responders. A patchy pattern with nonspecific distribution was seen in eight patients, of which four were responders.
"Myocardial edema by triple inversion recovery sequencing was seen in 6 patients, all having diffuse epicardial pattern of enhancement matching the delayed enhancement pattern," Anand and colleagues reported. "When comparing different treatment groups, among those treated for GD (n = 31), 12 of 21 (57%) responders had LGE and 4 of 10 nonresponders had LGE (p = 0.37), a pattern similar to the broader population. Among those not treated for GD (n = 6), 1 of 2 responders had LGE and 1 of 4 responders had LGE (p = 0.5)."