CT texture analysis of standard contrast-enhanced CT images in patients with colorectal liver metastases prior to treatment is showing promising results in predicting outcome prior to liver resection. TexRAD research software was used for analysis.
In oncology, imaging plays a crucial role during the patient's management. A radiologist looks at the images of the tumor and tries to perceive the patterns and report whether the tumor appears to be heterogeneous or homogeneous. This process depends heavily on the experience and knowledge of the radiologist and hence is quite subjective. Simple routine measurements, e.g., tumor size and attenuation, have proven to be useful in assessing tumors. However, these measurements do not provide key information about the heterogeneity or complexity of the tumor, which is a hallmark of cancer biology.
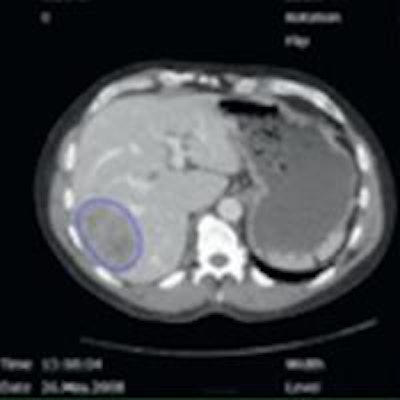
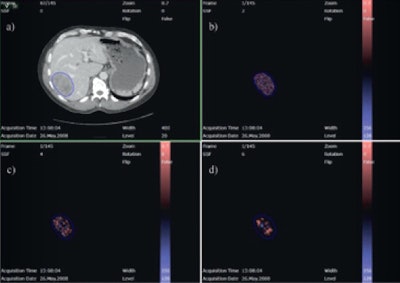 Evaluation of a colorectal liver metastasis with TexRAD CT texture analysis. Definition of a region of interest (a), and textures at different filtration levels corresponding to fine, medium, and coarse (b, c, d). All images courtesy of Dr. Anselm Schulz.
Evaluation of a colorectal liver metastasis with TexRAD CT texture analysis. Definition of a region of interest (a), and textures at different filtration levels corresponding to fine, medium, and coarse (b, c, d). All images courtesy of Dr. Anselm Schulz.Texture is a key component of image analysis, and texture analysis of these tumor images can provide vital heterogeneity information in a standardized and objective manner. In particular, texture analysis can measure and quantify heterogeneity within a tumor, potentially caused by local variation in hypoxia, necrosis, metabolic activity, proliferation, and neovascularization (different hallmarks of cancer). Differences in heterogeneity could be associated with different tumor disease severity, prognosis, or response to treatment.
TexRAD (www.texrad.com, part of Feedback, Cambridge, U.K.) is commercially available research software that employs a patented image filtration histogram-based texture analysis technique. The filtration step extracts and enhances features (or objects) of different sizes, number, and density variation; key image components of what makes a tumor appear heterogeneous or homogeneous. This filtration step is followed by quantification of the distribution of the histogram through statistical parameters, such as mean gray-level intensity, standard deviation, kurtosis, skewness, and entropy, which could reflect the different aspects of heterogeneity.
TexRAD has initially been applied in CT (with and without contrast) and recently in MRI (different sequences). It has also been evaluated in different tumor applications (lung, colorectal, primary and occult liver metastases, breast, esophagus, prostate, gliomas, head and neck, lymphoma, kidney pancreas, sarcoma, and melanoma) as an imaging biomarker (prognosis, disease severity, and response evaluation).
TexRAD is user-friendly software and can easily be integrated into hospital PACS/IT systems and employed in routine clinical practice. We have studied the application of TexRAD on standard contrast-enhanced CT images to predict the clinical outcome of patients with resectable colorectal liver metastases (CRLM) prior to treatment. Seventy-two patients were treated with open liver resection between July 2007 and December 2009. According to our exclusion criteria, 26 patients were excluded due to previous liver resection, which resulted in our final study population of 46 patients. The study was performed in collaboration with Dr. Balaji Ganeshan, senior imaging scientist at University College London, U.K. and one of the main inventors of TexRAD technology.
The optimal parameter for overall survival (OS) was mean gray-level intensity with lower unfiltered values (threshold 58.8) indicating shorter survival (1,306 vs. 1,873 days), which corresponded to a hazard ratio of 6.6. The best parameter for predicting recurrence-free survival (RFS) was entropy with higher values at fine texture scale (threshold 4.9) indicating shorter recurrence-free survival (557 vs. 1,358 days), which corresponded to a hazard ratio of 3.3.
Both mean gray-level intensity and entropy were significant in univariate and multivariate analysis. The CT texture analysis showed promising results in predicting outcome prior to liver resection for CRLM, with the potential to be a novel and independent preoperative predictor of both OS and RFS.
As a future prospect, TexRAD could potentially play a vital role in "personalized/precision medicine" for optimizing individual patient management, for example to identify patients who will benefit most from surgical resection of their colorectal liver metastases.
Dr. Anselm Schulz is a radiologist at the Department of Radiology and Nuclear Medicine at Oslo University Hospital in Norway.
Note: This article was originally published in ECR Today on 8 March 2015.
Copyright © 2015 European Society of Radiology