An MRI-based prostate cancer computer-aided detection (CAD) system is scoring high marks for detecting intermediate-to-aggressive prostate cancers noninvasively, Dutch investigators have reported.
Their study of 130 prostate cancer patients yielded sensitivity of more than 80% -- about the same as radiologists -- with a low corresponding false-positive rate. When the CAD is used as a second reader in combination with radiologists, sensitivity approaches 100%, according to the group from Radboud University Medical Center in Nijmegen, the Netherlands.
"We were able to show that CAD has about the same level of performance as a group of radiologists, and we have shown that CAD provides complementary information," said Henkjan Huisman, PhD, in a talk at the RSNA 2014 meeting. "We find more cancers and we find less insignificant cancers, reducing biopsies."
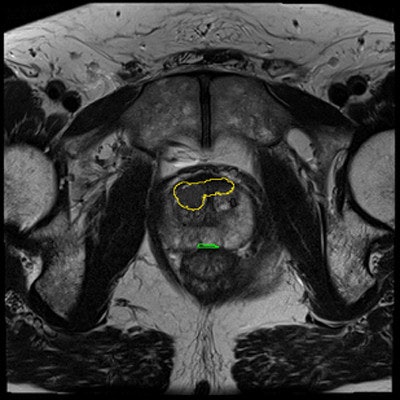
Prostate cancer has been especially hard to diagnose because the gold-standard diagnostic method is unreliable.
"For the last 20 years prostate cancer been detected by the [prostate-specific antigen test] PSA," Huisman said. "If you had a high PSA the only option was prostate biopsy. It was not very specific, not very sensitive, and so it missed cancers, so we need something else."
In many ways, prostate MRI has fitted the bill, offering a target for biopsy, but diagnosis can still be time consuming and unreliable in some cases. MRI can reduce the need for biopsy by 80%, but there are still cases where individuals with high PSA results undergo MRI, but the cancer cannot be found.
 Henkjan Huisman, PhD, assistant professor of radiology at the diagnostic image analysis group at the department of radiology, Radboud University Medical Center.
Henkjan Huisman, PhD, assistant professor of radiology at the diagnostic image analysis group at the department of radiology, Radboud University Medical Center.Something better still is needed, and that is prostate cancer CAD. A few years ago PhD student Geert Litjens, Huisman, and colleagues developed a fairly simple but functional prostate cancer CAD that performed "pretty well," Litjens said. The group has since updated it, expanding it to include zonal registration of tumors and multiatlas reference capabilities. There is feature extraction that looks for quality and texture features and a voxel classifier for tumor candidate detection and segmentation as well.
The new feature combination was tested on 348 cases with cross validation, and showed 82% sensitivity and only one false-positive per case.
"Now, using the same system we were looking for a way to validate the system," Litjens said. They settled on a strategy to apply the CAD to datasets and use logistic regression analysis to arrive at a standalone CAD score, a radiologist score, and a combined score.
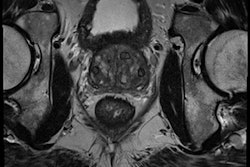
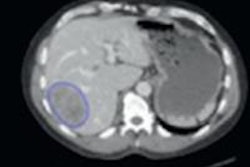
The new study used two consecutive cohorts: one (347 patients) for training and one for prospective evaluation of the CAD (130 patients). With both groups, analysis was followed by MRI-guided biopsy and pathology. MRI was performed on a 3-tesla MRI scanner (Magnetom, Siemens Healthcare) without use of an endorectal coil.
 PhD candidate Geert Litjens from the diagnostic image analysis group at Radboud University Medical Center.
PhD candidate Geert Litjens from the diagnostic image analysis group at Radboud University Medical Center.One of ten radiologists with a wide range of experience levels (1-20 years) interpreted both cohorts prospectively based on prostate imaging reporting and data system (PI-RADS 2.0) guidelines. Finally, logistic regression analysis was used to combine the CAD and radiologist scores into a combined score.
The CAD works in two stages: voxel classification and a subsequent candidate segmentation and classification stage, he noted. CAD features include quantified T2 measures, apparent diffusion coefficient (ADC), pharmacokinetics, texture, and anatomic characteristics. Both receiver operating curve (ROC) and free-response ROC (FROC) were used for analysis.
The group measured diagnostic performance in detecting intermediate-to-high-grade cancers on the CAD system alone, then with the radiologist alone, and then radiologist and CAD as a second reader, measuring specificity for the different PI-RADS thresholds. Bootstrapping assessed the significance of the findings.
The results in 130 patients showed high sensitivity at a low false-positive rate. CAD performed at about the level of radiologists, and it provides complementary information so that combined, the result is significantly better, according to Litjens.
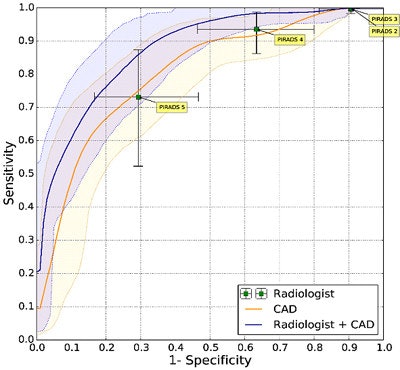 Prostate CAD scheme shows high detection rate, and results can be improved when CAD is used as a second reader. In 130 prostate cancer patients, CAD and radiologists were shown to have similar sensitivity. Simulating the addition of CAD improved sensitivity from 0.78 to 0.87 (indolent versus aggressive tumors). It improved sensitivity from 93% to 98% at PI-RADS 4.
Prostate CAD scheme shows high detection rate, and results can be improved when CAD is used as a second reader. In 130 prostate cancer patients, CAD and radiologists were shown to have similar sensitivity. Simulating the addition of CAD improved sensitivity from 0.78 to 0.87 (indolent versus aggressive tumors). It improved sensitivity from 93% to 98% at PI-RADS 4.For aggressive cancers at PI-RADS levels four and five, "we able to show that CAD improves the area under the curve from 0.78 to 0.87," Huisman said. "If a radiologist were to use CAD, then the sensitivity would increase 98%, which would really allow them to not do any biopsies for PI-RADS, which is clinically very relevant."
A larger prospective evaluation is in the works to further evaluate the CAD system, and the group has recently received funding to integrate the CAD into routine practice, which will allow further fine-tuning and assessment.
"We hope that we can go on through and show that CAD can really improve prostate MR diagnosis, and that CAD can help further reduce unnecessary biopsies," he said.
Litjens concluded that commercialization efforts are definitely planned. "We are actively looking at options for commercialization, but no concrete plans or timeline yet," he wrote in an email.