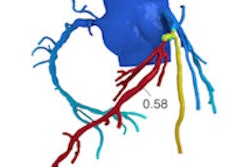
Can calculating contrast enhancement at coronary CT angiography (CCTA) replace the need for additional tests to determine the functional significance of coronary stenosis? Several approaches have been developed to do this, but a new one known as corrected contrast opacification (CCO) looks promising for potentially replacing perfusion imaging using MRI or SPECT to assess cardiac function after CCTA, say researchers from the Netherlands.
The researchers from the University of Groningen aimed to determine if they could assess the functional significance of a coronary artery stenosis at CT without need for an additional, invasive test such as perfusion MRI or SPECT.
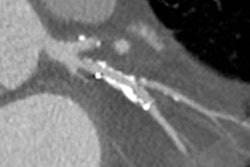
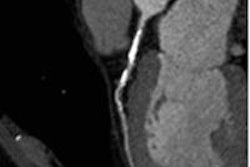
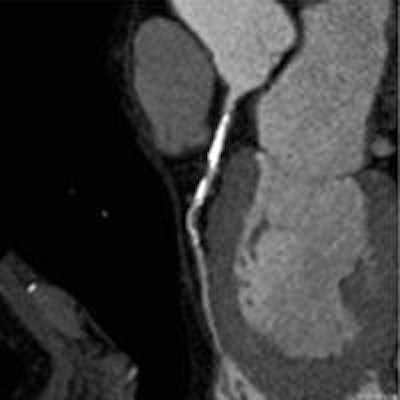
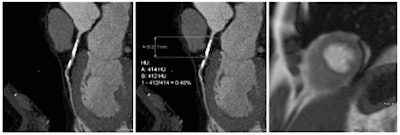 Coronary artery stenosis without hemodynamic significance: CCTA images are at left and center, while MRI (the reference standard for myocardial ischemia) is on the right. Images courtesy of Dr. Rozemarijn Vliegenthart.
Coronary artery stenosis without hemodynamic significance: CCTA images are at left and center, while MRI (the reference standard for myocardial ischemia) is on the right. Images courtesy of Dr. Rozemarijn Vliegenthart.In the study, each of the 60 asymptomatic patients underwent CCTA and CCO analysis in addition to adenosine perfusion MRI (APMRI). In the analysis of 169 coronary artery stenosis, CCO showed a strong association with functional stenosis at APMRI, but was not correlated to the presence of anatomic stenosis at CCTA, the group reported at ECR 2014.
"There was a significant difference in CCO between stenosis causing ischemia compared to those that do not cause ischemia," said Dr. Martijn den Dekker in his talk.
Coronary heart disease kills nearly 400,000 people every year, according to the U.S. Centers for Disease Control and prevention. Coronary artery stenosis can be readily detected using noninvasive imaging modalities such as CT or MRI, but these tests don't address its impact on cardiac function -- a question that is best addressed with perfusion imaging, usually MRI or SPECT myocardial perfusion imaging.
A number of groups are working on CTA-based approaches for evaluating the functional significance of stenosis based on contrast attenuation in the coronary lumen, wrote study co-author Dr. Rozemarijn Vliegenthart in an email to AuntMinnieEurope.com.
"For example, transluminal attenuation gradient assessment is a similar approach," she wrote. "The CCO and similar approaches are based on differences in contrast attenuation across the coronary artery and across the coronary stenosis, adjusted for aortic enhancement. One difference with the CT fractional flow reserve (FFR) software process is that on site evaluation is possible, and there is no need to send data to a remote lab."
CCO "estimates the effect of a stenosis by measuring the Hounsfield contrast attenuation before and after a stenosis and corrects this for time using attenuation in the descending aorta," den Dekker explained. "This might substitute for functional imaging and can be added to the anatomical information of CCTA."
The study looked at the association between CCO and inducible ischemia as detected with adenosine perfusion MRI. In a substudy of the GROUND2 trial in which 115 patients were included since 2009, the research team for this analysis looked at 60 cardiac asymptomatic patients with proven extracardiac arterial disease (78% men, mean age 64.4 ± 7.7 years).
CCTA plus adenosine perfusion MRI
 Dr. Martijn den Dekker.
Dr. Martijn den Dekker.
All patients underwent baseline CCTA using a dual-source scanner and APMRI on a 1.5-tesla MRI scanner. For this study, the investigators defined ischemia as a reversible perfusion defect at APMRI.
CCTA images were acquired on a dual-source CT scanner (Somatom Definition, Siemens Healthcare) using retrospective ECG gating, 32 x 0.6-mm collimation, 0.4-mm slices, field-of-view 205 mm, and gantry rotation time of 330 ms, with tube voltage and current set by patients weight, den Dekker said.
To assess the arteries, the group measured luminal CT attenuation values (in Hounsfield Units) in the proximal, middle, and distal segments of each coronary artery, using a dedicated workstation (Aquarius Intuition Edition, TeraRecon). Additional measurements (two proximal and two distal) were performed in arteries with significant (> 50%) lumen stenosis. CCO was then calculated by dividing coronary CT attenuation by descending aorta CT attenuation at equal level.
CCO was calculated by dividing the coronary measurements by the aorta attenuation at the same level, and the group calculated decreases in CCO across the coronary artery and across stenoses, comparing them with presence of inducible ischemia on APMRI using regression analysis.
Patients were divided into two groups: those with (n = 7) and those without ischemia (n = 53) on APMRI. Differences between the groups were assessed using the Mann-Whitney U test, the chi-square test, the Kruskall-Wallace test, and the Spearman correlation coefficient.
"Although the two groups were not similar in size, there was only a significant difference in diabetes (APMRI negative, 5.7%; APMRI positive, 28.6%) -- otherwise the parameters were comparable for both groups," den Dekker said.
In all, the group found 169 coronary stenoses. Seven patients had eight perfusion defects on APMRI, with 11 stenoses in the corresponding coronary arteries. Looking at all 180 possible coronary arteries for analysis, 28 were excluded due to small vessel size, artifact, or severe calcification. Of the 152 remaining arteries, 65 were without significant stenosis, including 13 arteries with stenosis between zero and 50%. Of the 87 coronary arteries with significant stenosis, only eight were causing ischemia (3 LAD, 1 LCX, 4 RCA).
CCO drops for ischemia
The median CCO for normal coronary arteries was 1.075 [25th, 75th percentiles: 0.978, 1.169] at the origin, and 1.021 [25th, 75th percentiles: 0.876, 1.142] at the distal segment. There is an underlying decrease in contrast attenuation between the proximal and distal segments, "and the reason for this is the time factor as the proximal and distal segments are not acquired in the same heartbeat," he noted.
The key to the study was the difference in CCO measurements across stenosis -- measurements that were significantly larger in patients with a perfusion defect causing hemodynamically significant stenosis (0.144 ± 0.112 m) compared with patients without perfusion defects (0.047 ± 0.104; p = 0.003).
But anatomically significant stenosis was another story: No CCO differences were seen between patients with anatomically significant stenosis (0.054 ± 0.116) and those without (0.052 ± 0.101; p = 0.89).
"When we measured the contrast attenuation before and after a significant stenosis there was no difference in those without ischemia, while a decrease [in contrast attenuation] was seen in stenosis with hemodynamic significance," den Dekker said. "There was also ... a difference in CCO between stenosis causing ischemia compared to those that do not cause ischemia -- while on CCTA there was no difference [between] anatomically significant and nonsignificant stenoses."
In conclusion, he said, "There is a strong association between CCO and hemodynamically significant stenosis, while this association was not seen for merely anatomically significant stenosis. So CCO could potentially exclude stenosis without hemodynamic significance from further clinical workup."
Will CCO do the job?
"At this moment, we only have first indications that CCO may be helpful to determine hemodynamic significance of a stenosis, but larger studies should confirm this," commented Vliegenthart, citing new research by Wong et al in the Journal of the American College of Cardiology, who compared an approach similar to CCO to invasive fractional flow reserve (FFR).
"If CCO will be reliable enough for decision-making, it will be an important addition to the common evaluation of coronary CTA in case of a stenosis, as a non-negligible proportion of anatomical stenoses do not cause ischemia," she wrote. "The calculation should not take much time. However, no cut-off values are known as yet, and values for stenoses with and without hemodynamic significance are still partly overlapping."