Breast cancer is the primary carcinoma among women worldwide, and imaging is indispensable not only in detecting lesions, but also in evaluating the efficacy of currently available therapies and warning of possible recurrence. In neoadjuvant treatment, functional imaging offers the option of assessing tumor response very early, eventually increasing chances of a successful outcome by guiding treatment. Radiologists can also assess residual disease after surgical biopsy at pathology with the help of both morphological and functional imaging. After therapy, annual examinations enable doctors to track any cancer reappearance.
Technological advances have improved patient care but radiologists are still facing a number of challenges -- starting with the absence of standardized protocols for functional imaging, which limits the benefits brought by diffusion imaging and spectroscopy. The high occurrence of false-positive results in findings from postsurgical breast imaging necessitates a savvy combination of different tools. Finally, potential risks induced by radiation exposure must be balanced against the benefits of detection of recurrent disease after therapy. These points and many more were debated by a panel of experts during a dedicated refresher course at the European Congress of Radiology (ECR) 2011 in Vienna.
The number of women treated with neoadjuvant treatments has significantly increased during the past few years, after prospective multicenter studies revealed that chemotherapy followed by surgery was not significantly different than surgery followed by chemotherapy for overall survival. Moreover, starting with the drug-based treatment increases the possibility of performing conservative surgery, and a pathologically complete response at surgery is a surrogate marker of survival.
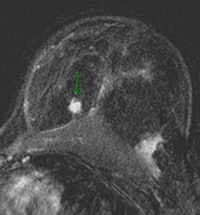 |
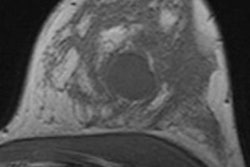
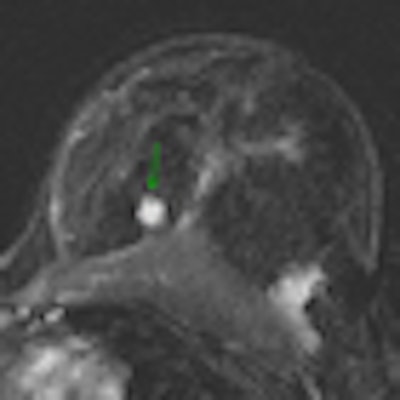
| Contrast-enhanced breast MRI (subtracted image three minutes after contrast medium injection). Patient with a diagnosis of invasive ductal carcinoma (ultrasound-guided biopsies). At surgery, no cancer was found. Ultrasound examination after surgery was unable to detect the lesion. At MRI, the lesion was well depicted (inner quadrants, deep location). Image courtesy of Dr. Anne Tardivon. |
Functional imaging enables assessment of tumor response as early as right after the first chemotherapy session, much earlier than any evaluation with morphological imaging. Diffusion-weighted imaging (DWI), combined with MRI, and MR spectroscopy have proved to be instrumental in assessing early tumor response. Both enable measurement of functional changes in a given tumor, focusing for instance on its extracellular space and chemistry -- e.g., by showing an abnormally high or reasonable number of choline metabolites with MR spectroscopy. DWI can act as a biomarker for tumor aggressiveness and monitor response to treatment by quantifying the apparent diffusion coefficient (ADC), which provides the diffusion coefficient value.
Unfortunately, the lack of uniformity among MR protocols limits the full use of these techniques. Since a patient is rarely examined on the same MR machine using the same imaging parameters, the coefficient value obtained on two machines is generally not the same, altering its reliability.
"These techniques are very promising but we have a real problem of application and reproducibility here," said Dr. Anne Tardivon, a radiologist at the Curie Institute in Paris and chair of the ECR course.
In the absence of existing guidelines, one could base the observation not on the value itself, but rather on the percentage of diminution between the examinations, she suggested.
With spectroscopy, few MR scanners used in routine are powerful enough to show the choline peak. New machines use 3-tesla instead of 1.5-tesla and are much more appropriate, but less available in practice.
Postsurgical imaging of the breast is also useful in determining whether cancer has been fully removed or not. Mammography helps to evaluate whether microcalcifications are left behind in the case of ductal carcinoma in situ (DCIS) and irradical operation. Ultrasound can show whether lesions are visible near the former site of the tumor, but its accuracy is limited because of hematoma and scar tissue.
Breast MRI is therefore the most accurate imaging modality in predicting residual disease, and specialists recommend performing an MR examination three to four weeks after surgery. However, radiologists must be aware of false-positive results triggered by enhancing scar tissue, Tardivon pointed out.
"They should especially consider that inflammations, which are common after surgery, might generate false positives because they create vascular hypermeability, and might take the contrast product used with MRI," she explained.
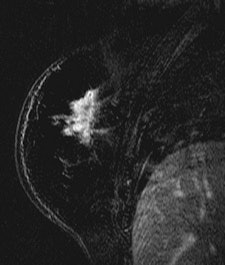 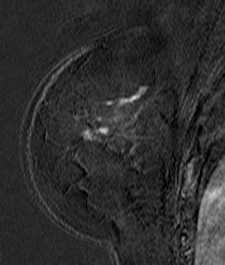 |
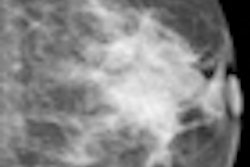
| Patient with breast cancer (invasive ductal carcinoma, grade II). MR-subtracted images (three minutes after contrast medium injection) before (left) and at the end (right) of the neoadjuvant chemotherapy. Initial staging: unifocal infiltrating cancer. After treatment, even if there is a decrease in size of the cancer, its long axis (RECIST) is unchanged with a multifocal shrinkage of the tumor. Unsuccessful first breast-conserving surgery, then mastectomy. Image courtesy of Dr. Anne Tardivon. |
Nuclear medicine tools, such as positron emitted mammography (PEM) and scintimammography, offer more specificity at this stage -- but here again false positives might occur, because of inflammatory processes.
How to follow up women who have been successfully treated also was an important topic of the course. European estimations for the rate of recurrence are between 0.4% and 0.8% each year, and this rate is higher than breast cancer incidence in the general population.
Mammography is the first modality used in detecting recurrent disease. It is usually carried out once a year, in some women for the rest of their lives. Ultrasound can also be used together with mammography, and MRI is prescribed for women with a high genetic risk as soon as they reach 30, and even if they have never had cancer.
However, specialists do not know which modality is the most appropriate -- based on the age at diagnosis and the type of cancer. There are no studies available on the subject yet. The American Cancer Society has given recommendations for MRI, estimating that there was insufficient evidence to recommend for or against MRI screening in patients treated for a breast cancer, including DCIS.
Finally, the financial costs of detecting recurrent disease and the identification of subgroups to determine the occurrence of examinations after cancer require expert consultation. One could, for instance, imagine changing the interval between mammography exams 10 or 15 years after cancer in older patients or adding MRI in younger patients with dense breasts in the first five years after cancer, Tardivon suggested.
Originally published in ECR Today March 6, 2011.
Copyright © 2011 European Society of Radiology