Multifunctional microbubble agents are showing great potential for medical applications, particularly superparamagnetic iron oxide (SPIO) nanoparticles, which are used as MRI contrast agents but can be combined with encapsulated microbubbles and provide ultrasound contrast plus targeted gene or drug delivery.
If the functionalities of such agents can be appropriately tailored by adjusting their structure and mechanical properties, they could simultaneously offer early diagnosis and effective treatment. However, there's currently a lack of knowledge regarding how integrating SPIOs into microbubbles affects their performance.
 Some of the Nanjing University researchers working on multifunctional microbubble agents.
Some of the Nanjing University researchers working on multifunctional microbubble agents.To address this, researchers from Nanjing University in China have synthesized a multifunctional agent by loading SPIO nanoparticles into albumin-shelled microbubbles and quantitatively analyzed how the SPIO concentration impacts the mechanical characteristics and dynamic performance of the microbubbles (Physics in Medicine and Biology, October 2014, Vol. 59:22, pp. 6729-6747).
"Our main motivation was to verify the feasibility of developing a multifunctional agent that can offer both satisfactory ultrasound/MR imaging sensitivity, and effective gene and drug delivery efficiency," Dong Zhang, director of Nanjing University's Institute of Acoustics, told medicalphysicsweb.
Mechanical assessments
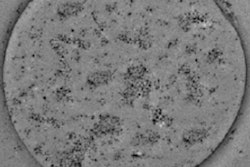
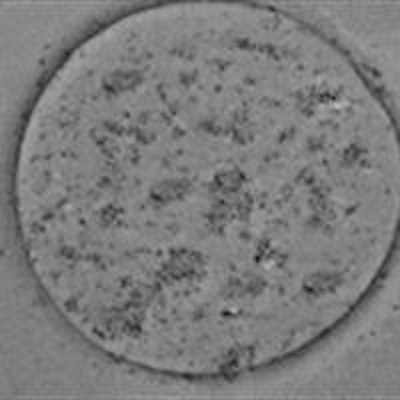
Zhang and colleagues first characterized the SPIO-containing microbubbles (SPIO-MBs) using transmission electron microscopy. Images of a microbubble showed a uniform surface, while an SPIO-MB showed nanometer-scale dark dots on the bubble surface, verifying successful integration of SPIOs. Atomic force microscopy (AFM) revealed that a typical microbubble had a relatively smooth surface, while the SPIO-MB was larger and had a rougher structure, due to embedding of SPIOs onto the bubble shell.
 (a) Transmission electron microscopy (TEM) image of microbubbles without the addition of nanoparticles; (b) TEM image of an SPIO-MB.
(a) Transmission electron microscopy (TEM) image of microbubbles without the addition of nanoparticles; (b) TEM image of an SPIO-MB.The researchers assessed the microbubble sizes seen for SPIO concentrations in microbubble suspension of 0.0, 19.6, 114.7, and 292.0 µg/mL (groups 1-4, respectively). The mean microbubble diameter increased with increasing SPIO concentration, reaching about 3.5 µm for group 4, and indicating that the SPIO-MBs are small enough to function as effective clinical agents.
They also performed AFM measurements to assess microbubble stiffness for the four groups. They saw that stiffness decreased with increasing SPIO concentration and, at a fixed concentration (group 4), decreased as the bubble diameter increased.
Imaging performance
The team next examined the impact of combining the two contrast agents on their respective imaging performance. MR images of SPIO-MBs in the four groups and SPIO solutions with corresponding molar iron concentrations showed that T2-weighted images became darker with increasing iron concentration. Only moderate contrast change was seen in T1-weighted images.
 T1- (a) and T2-weighted (b) MR images for: (I) degassed water; (II) SPIOs with 0.01688 mM iron concentration; (III) SPIOs with 0.0985 mM iron concentration; (IV) SPIOs with 0.252 mM iron concentration; (V) SPIO-MBs with no iron; (VI) SPIO-MBs with 0.01688 mM iron concentration; (VII) SPIO-MBs with 0.0985 mM iron concentration and (VIII) SPIO-MBs with 0.252 mM iron concentration.
T1- (a) and T2-weighted (b) MR images for: (I) degassed water; (II) SPIOs with 0.01688 mM iron concentration; (III) SPIOs with 0.0985 mM iron concentration; (IV) SPIOs with 0.252 mM iron concentration; (V) SPIO-MBs with no iron; (VI) SPIO-MBs with 0.01688 mM iron concentration; (VII) SPIO-MBs with 0.0985 mM iron concentration and (VIII) SPIO-MBs with 0.252 mM iron concentration.Calculating the ratio of the transverse to longitudinal relaxivity, an indicator of whether a contrast agent is most suitable for enhancing T1- or T2-weighted images, showed that SPIO-MBs may be better negative MRI contrast agents than regular SPIOs.
To investigate the impact of the SPIOs on the microbubbles' ultrasound imaging capability, the researchers imaged a gel phantom containing a 3 x 3 x 3 cm testing chamber. Microbubbles samples in each group were diluted 30 times and injected into the chamber. Compared with ultrasound images of degassed water, images of solutions with higher SPIO concentration clearly showed hyperechoic regions.
The averaged contrast-to-tissue ratios were -0.59, 5.89, 8.97, 11.04, and 12.844 dB, for degassed water and group 1 to 4 samples, respectively. The authors note that while microbubbles alone achieved significant contrast, this was enhanced further as the concentration of loaded SPIOs increased. "The results suggest that the combined SPIO-MB agents exhibited better performance for both ultrasound and MR imaging that the single contrasts," Zhang noted.
Therapeutic agent
As the SPIO-MBs were also designed for drug/gene delivery, Zhang and colleagues evaluated their impact upon ultrasound-facilitated vascular endothelial growth factor (VEGF) transfection. They examined human embryonic kidney cells mixed with branched polyethylenimine:VEGF complexes, and cells mixed with the complexes plus microbubbles in groups 1-4. Samples were exposed to 1 MHz ultrasound at acoustic driving pressures of 0, 0.05, 0.2, 0.5, and 1.0 MPa.
For all samples, the researchers assessed cell viability and VEGF protein levels in the cell suspensions. They also used passive cavitation detection to determine the inertial cavitation energy during ultrasound exposure (the "inertial cavitation dose"), because inertial cavitation plays an important role in gene/drug delivery into cells.
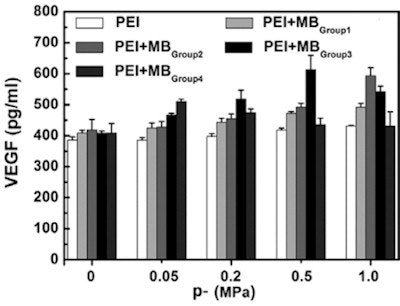 Dependence of transfection efficiency on acoustic driving pressure for SPIO-MBs with varied SPIO concentrations. 1 MHz ultrasound exposures were performed at fixed 20-cycle pulse length and 250 Hz PRF. The total treatment time for each sample was 20s. The figure plots results for five replicated measurements.
Dependence of transfection efficiency on acoustic driving pressure for SPIO-MBs with varied SPIO concentrations. 1 MHz ultrasound exposures were performed at fixed 20-cycle pulse length and 250 Hz PRF. The total treatment time for each sample was 20s. The figure plots results for five replicated measurements.The inertial cavitation dose generally increased with increasing pressure and higher SPIO concentrations. Without ultrasound exposure, adding microbubbles resulted in only a slight decline in cell viability, suggesting that the SPIO-MBs have relatively low cytotoxicity. After ultrasound exposure, cell viability was significantly lowered with increasing pressure and SPIO concentration, to a maximum reduction of 56.5%.
The transfection efficiency results were more complex. With no ultrasound exposure, there was no obvious enhancement observed when adding microbubbles. With ultrasound, adding pure microbubbles (group 1) increased the transfection efficiency compared with samples mixed with polyethylenimine:VEGF only.
Group 2 SPIO-MBs significantly improved transfection, more so at higher pressures. For group 3 SPIO-MBs, however, transfection efficiency initially increased with increasing pressure, reached a peak at 0.5 MPa and then decreased. The highest SPIO concentration (group 4 SPIO-MBs) showed a similar pattern, but with an earlier peak at 0.05 MPa. Overall, the largest VEGF transfection was achieved by using SPIO-MBs with a concentration of 114.7 µg/ml (group 3) at an acoustic pressure of 0.5 MPa.
"The results show that embedding SPIOs into the shells of normal contrast agent microbubbles can enhance their ultrasound and MRI performance," Zhang said. "However, optimized VEGF transfection can only be achieved [using an] appropriate SPIO concentration. In other words, it's important to get further information about the structure--property relationship of these multimodal microbubbles to make them satisfy the different requirements of particular applications."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.