In a bid for clarity, the European Society of Breast Imaging (EUSOBI) has issued new recommendations to standardize patient management and reduce unnecessary imaging and invasive procedures in cases of unilateral axillary lymphadenopathy -- a frequent mild side effect of COVID-19 vaccination.
"Since the rollout of COVID-19 vaccines is rapidly proceeding, radiologists will increasingly encounter in their practice COVID-19 vaccination-induced lymphadenopathy detected by breast imaging," noted Dr. Simone Schiaffino and Prof. Francesco Sardanelli from IRCCS Policlinico San Donato, San Donato Milanese, Italy, and colleagues from EUSOBI.
"The emergence of new SARS-CoV-2 variants and the unclear durability of vaccine-induced immunity will likely lead to re-vaccination or to the administration of new vaccines, further extending this issue," they wrote in an article posted on 20 August by Insights into Imaging.
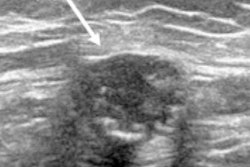
 Ultrasonography of the left axilla shows an enlarged 17 mm reactive lymph node in a 45-year-old woman about a week after receiving the firrst dose of the Vaxzevria COVID-19 vaccine (Astra Zeneca). Note the asymmetrical cortical thickening (white arrow) associated with a well-represented central fatty hilum. All images courtesy of the EUSOBI and Insights into Imaging.
Ultrasonography of the left axilla shows an enlarged 17 mm reactive lymph node in a 45-year-old woman about a week after receiving the firrst dose of the Vaxzevria COVID-19 vaccine (Astra Zeneca). Note the asymmetrical cortical thickening (white arrow) associated with a well-represented central fatty hilum. All images courtesy of the EUSOBI and Insights into Imaging.In the article, the EUSOBI group provides 10 recommendations about general issues (numbers 1 and 2 below); asymptomatic subjects, including women attending screening programs (number 3); cases with symptoms or imaging-detected findings (number 4-9); and complex cases (number 10):
- In patients with a previous history of breast cancer, vaccine injection (both doses for two-dose vaccines) should be performed in the contralateral arm or in the anterolateral thigh.
- COVID-19 vaccination data (vaccination status, date, dose, injection site) of all patients presenting for breast imaging with any modality should be collected and made available to radiologists, including the cases of breast imaging performed for cancer staging and of follow-up imaging examinations.
- Breast examinations should be preferentially performed before the first dose of a COVID-19 vaccine or at least 12 weeks after the injection. For vaccines with a two-dose schedule, the 12-week rule applies from the day of the second injection.
- In patients newly diagnosed with breast cancer, all necessary breast imaging examinations with any modality must be performed without any delay due to vaccination, taking into consideration the risk of false positive lymph node findings.
- The contralateral axilla and both breasts should be clinically examined using appropriate imaging to exclude malignancy in all patients with axillary symptoms and in all cases of imaging-detected unilateral axillary lymphadenopathy before vaccination or at least 12 weeks after.
- In patients with or without previous breast cancer history, imaging-detected suspicious axillary lymphadenopathy contralateral to the vaccination side should be managed according to standard workup protocols, including, when necessary, tissue sampling.
- In patients without breast cancer history and no suspicious breast imaging findings, imaging-detected unilateral axillary lymphadenopathy on the same side of recent COVID-19 vaccination (i.e., within 12 weeks) should be managed according to the clinical setting. In asymptomatic patients, it should be classified as a benign finding (BI-RADS 2) and no further workup should be pursued. In case of patients reporting symptoms of axillary lymphadenopathy more than 12 weeks after vaccination, ultrasound examination of the axilla is recommended. In patients with axillary symptoms, incidental unilateral axillary lymphadenopathy ipsilateral to the vaccination side without any suspicious finding in the breast should be classified as a probably benign finding (BI-RADS 3), requiring a 12-week follow-up. In case of persistent suspicion at this 12-week follow-up, ACR BI-RADS recommendations for the management of axillary lymphadenopathy should be followed, with further workup including, when necessary, tissue sampling.
- In patients without breast cancer history, incidental unilateral axillary lymphadenopathy after COVID-19 vaccination coupled with ipsilateral suspicious findings in the breast at any imaging modality should be managed according to clinical practice, including biopsy when appropriate.
- In patients with personal breast cancer history, lymphadenopathy after vaccination should be interpreted considering the time since vaccination and overall nodal metastatic risk (cancer type, location, stage, etc.). For patients at low risk of axillary or supraclavicular nodal metastases in whom the lymphadenopathy is much more likely due to the vaccination than to the underlying neoplasm (considering time frame, pain, type, and location of cancer), a cautious management strategy without default follow-up imaging is appropriate. Short-interval follow-up imaging with ultrasonography (with at least a 12-week delay) may be performed in patients with higher risk of metastatic lymphadenopathy (e.g., breast cancer, head and neck cancer, upper extremity/trunk melanoma, or lymphoma). Node biopsy should be considered in the setting of high nodal metastatic risk when immediate histopathologic confirmation is necessary for timely patient management.
- All complex or unclear cases (e.g., axillary lymphadenopathy ipsilateral to the cancer and the side of vaccination within 12 weeks after vaccination in patients with previous bilateral breast cancer; vaccinations performed on different sides) should follow a personalized management, considering the risk of malignant lymphadenopathy, opting for tissue sampling when appropriate after multidisciplinary team discussion.
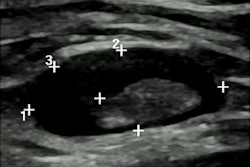
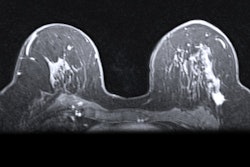
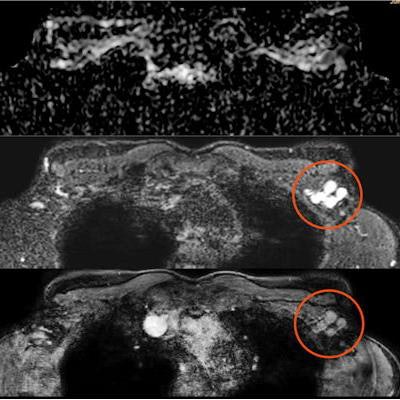
 Screening mammography performed in a 44-year-old woman with a positive family history for breast cancer (mother and aunt), bearing implants for aesthetic purposes. Mammography (A) was considered negative. Breast ultrasonography was also performed because of her family history and high breast density (ACR category D). While ultrasonography was negative for both breasts, multiple round, enlarged, hypoechoic lymph nodes (measuring up to 1 cm in axial diameter), with a thickened (< 3 mm) cortex, were seen in the left axilla (B). There were no skin changes and there was no history of any infection or trauma. On the right side, axillary lymph nodes were normal. Because of her family history and the presence of breast implants, MRI was performed (C = apparent diffusion coefficient map; D = T2-weighted short-time inversion recovery; E = fat-saturated contrast-enhanced T1-weighted gradient-echo). No suspicious mass or nonmass lesions were seen in both breasts. Implants displayed no signs of rupture (not shown). In the left axilla, multiple enlarged lymph nodes were well visible in D and E (red circles); on the apparent diffusion coefficient map (C), they mainly exhibited low signal (restricted diffusivity). When an ultrasound-guided biopsy of the most suspicious lymph node was proposed, the patient mentioned that she had had a Comirnaty COVID-19 vaccination (Pfizer-BioNTech) one week before in the left arm. The attending radiologist was more than surprised to hear this, as at that time, a COVID-19 vaccination was only administered to people older than 70 years. Follow-up performed four weeks after the second vaccination was negative and showed no residual enlarged lymph nodes.
Screening mammography performed in a 44-year-old woman with a positive family history for breast cancer (mother and aunt), bearing implants for aesthetic purposes. Mammography (A) was considered negative. Breast ultrasonography was also performed because of her family history and high breast density (ACR category D). While ultrasonography was negative for both breasts, multiple round, enlarged, hypoechoic lymph nodes (measuring up to 1 cm in axial diameter), with a thickened (< 3 mm) cortex, were seen in the left axilla (B). There were no skin changes and there was no history of any infection or trauma. On the right side, axillary lymph nodes were normal. Because of her family history and the presence of breast implants, MRI was performed (C = apparent diffusion coefficient map; D = T2-weighted short-time inversion recovery; E = fat-saturated contrast-enhanced T1-weighted gradient-echo). No suspicious mass or nonmass lesions were seen in both breasts. Implants displayed no signs of rupture (not shown). In the left axilla, multiple enlarged lymph nodes were well visible in D and E (red circles); on the apparent diffusion coefficient map (C), they mainly exhibited low signal (restricted diffusivity). When an ultrasound-guided biopsy of the most suspicious lymph node was proposed, the patient mentioned that she had had a Comirnaty COVID-19 vaccination (Pfizer-BioNTech) one week before in the left arm. The attending radiologist was more than surprised to hear this, as at that time, a COVID-19 vaccination was only administered to people older than 70 years. Follow-up performed four weeks after the second vaccination was negative and showed no residual enlarged lymph nodes.Further research and adherence to evidence-based recommendations are paramount to standardize the management of findings, avoiding unnecessary additional imaging, and invasive procedures, the authors concluded.