Ultrahigh-field (UHF) MRI -- and specifically 7-tesla MRI -- now is a clinically viable method for studying knee cartilage, thanks in large part to the development of a custom-built volume coil, according to researchers from Pisa University Hospital in Italy.
Data from Dr. Giacomo Aringhieri and colleagues revealed a significant correlation between both the increase in the T2 and T2* maps for mean values and subjects' age. They found that using a surface coil makes a big difference, and the device represents an important technological innovation for UHF MRI of the knee.
The Pisa group sought to optimize cartilage-dedicated sequences for in vivo articular cartilage imaging at 7-tesla MRI and to investigate the diagnostic potential of UHF MRI in detecting early changes of the articular cartilage related to physiological aging, focusing on the feasibility and the results of in vivo T2 and T2* mapping.
Optimum technique
A total of 28 healthy subjects (n = 20, < 50 years, mean age 37.75 ± 8.84; n = 8, > 50 years, mean age 59.75 ± 6.38) were recruited for the study. Inclusion criteria were the absence of clinical symptoms and no history of previous knee surgery or significant knee trauma. All the subjects underwent a knee examination on a 7-tesla whole-body system.
"For sequence optimization we started with ex vivo examinations of a pig leg, and human femoral and tibial parts (after prosthetic implants) using the head coil, then we began studying knees of healthy volunteers," Aringhieri and colleagues explained in an e-poster presentation at ECR 2017 in Vienna.
At first, they used a surface coil, called Rossella, to evaluate the knee, but only the anterior compartment could be studied with this device, so they replaced it with an eight-channel volume coil, called Kneena, created in their own laboratory. The new coil had a different field-of-view, and it allowed them to explore the whole knee and exploit parallel imaging.
The team's 7-tesla optimized sequences for the cartilage were as follows:
- FIESTA-C (constructive interference into steady state) for cartilage morphology and segmentation because of the optimal contrast between anatomic structures, including liquid, fat, muscle, and cartilage
- Multiecho-spin-echo-T2 for the T2 maps
- 3D susceptibility-weighted imaging for the T2* maps
Also, 3D-fast spoiled gradient echo (FSPGR) fat-saturated, 3D-steady state free precession (SSFP, coherent-oscillatory), and gradient-recalled echo (GRE)-2D-multiecho recombined sequences were acquired. The highest spatial resolution achieved was 136 µm.
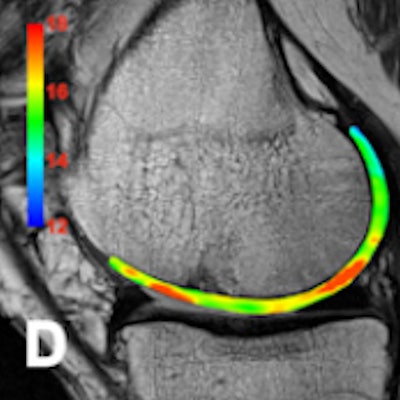
No significant adverse events were observed; two subjects complained of metallic taste and transient paresthesia. Cartilage segmentation was performed with an open source application called ITK-SNAP (3.2 version). The average T2 maps values were 26.5 msec ± 1.5 (< 50 years) and 29.5 msec ± 1.7 (> 50 years). The mean T2* maps values were 21.1 msec ± 2.2 (< 50 years) and 25.6 msec ± 1.6 (> 50 years).
Progress on intraoperative MRI
Also at ECR 2017, Spanish researchers presented new information about the role of high-field intraoperative MRI in the management of neuro-oncologic patients.
"The implementation of an intraoperative MRI unit requires a large economic investment and represents a great infrastructural challenge that takes months or even years to plan," noted Dr. Pablo Bartolomé and colleagues in the department of radiology at Universidad de Navarra, Clínica Universidad de Navarra in Pamplona. "It helps the neurosurgeons to overcome brain shift, and improves extent of resection in glioma patients, allows for the early detection of intraoperative complications, and reduces the incidence of second-look surgeries and the number of postoperative imaging studies."
At their hospital, a total of 59 patients underwent intraoperative MRI between February and September 2016. In patients with low-grade glioma, it helped extend tumor resection in nine out of 11 cases (83%), and in patients with high-grade glioma, it helped extend tumor resection in 13 out of 25 cases (52%).
Maintaining sterility in the MRI suite and ensuring patient safety have been the major challenges. Overall, the day-to-day functioning of a high-field intraoperative MRI requires the contribution of a neuro-oncology multidisciplinary team that includes neurosurgeons, radiologists, anesthesiologists, and nurses, according to Bartolomé and colleagues.
They use a 3-tesla unit (Magnetom Skyra from Siemens Healthineers) located in a room opposite the neurosurgical operating room. The imaging protocol is standardized, using a localizer, T1-weighted 1 mm slice thickness MP-RAGE (magnetization-prepared rapid gradient-echo) sequence, and diffusion-weighted and diffusion tensor sequences. The unit is not a dedicated device, and when it is not needed for neurosurgical procedures, it is used for diagnostic purposes.
"The neurosurgical procedure is performed on a special MRI-compatible table fitted with wheels," they explained. "The table is detached and the patient is transferred to the MRI room. The table is then fixed to the MRI equipment to perform the study. The patient is anesthetized the whole time, and is continuously monitored."
The radiologist is present during the whole procedure, and supervises imaging protocol and quality. After the images are obtained, the radiologist analyzes the data in real-time and sends the results to the surgeons so they can decide on the best course of action.
To view the full selection of clinical cases and images presented at ECR 2017, click here for the e-poster by the Pisa group, and click here for the presentation by the Pamplona team.
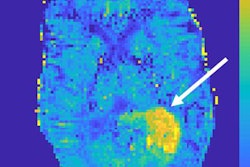
Editor's note: On the home page is a 7-tesla sodium (Na-23) MR image of a patient eight years after autologous osteochondral transplantation. Biochemical methods can reveal alterations in the normal structure of the cartilage transplant. Chemical exchange saturation transfer and sodium imaging can only be performed with 7-tesla MR scanners. Image courtesy of Dr. Fritz Schick, head of the department of experimental radiology at Tübingen University Hospital in Germany.