There is no consistent approach to renal function assessment prior to administration of gadolinium-based contrast agents, even though international guidance does exist and consistency is vital to minimize risk and ensure safe practice, a new U.K. survey has found.
"Point-of-care technology may offer the opportunity to standardize practice, particularly when used in conjunction with a prescreening questionnaire, but further evidence is required to support implementation," Ruth Clarke, consultant radiographer at Mid Yorkshire Hospitals National Health Service (NHS) Trust, told delegates at the recent U.K. Radiological Congress (UKRC) in Liverpool.
Clarke and her colleagues sought to identify current U.K. practices for the identification and minimization of risk in outpatients referred for contrast-enhanced MRI with gadolinium-based contrast agents (GBCAs). They invited radiology departments to complete an online survey about local policies and guidance followed, focusing on practice for checking renal function and patient management pre- and post-GBCA administration.
A total of 68 out of 174 sites replied, making a response rate of 39.1%, and 82.3% of sites confirmed a policy was in place for GBCA administration. The majority indicated alignment with at least one national and/or international guideline (n = 49/56; 87.5%). There were variations between responses, but the most common was guidance from the Royal College of Radiologists (RCR).
More than 41% (28 sites) checked blood test results for all patients, whereas 31 sites (45.6%) only checked those with known renal dysfunction or identified as high risk for renal impairment.
"There was no consensus on acceptable timescales for tests prior to contrast, or who was responsible for organizing bloods," Clarke noted. "Where blood tests were being performed, the checking of the results was often done on the day of the scan, nullifying any gain from performing blood tests in advance."
Six sites used point-of-care blood tests if recent results were unavailable. Six hospital groups were evaluating point-of-care devices, and 12 sites had rejected it as an adjunct to the pathway. The most common reason for this was cost. Lack of support from pathology, reliability and accuracy issues, and incompatibility of measures were also cited. Concern that the immediacy of point-of-care results could reduce imaging capacity was cited by three sites.
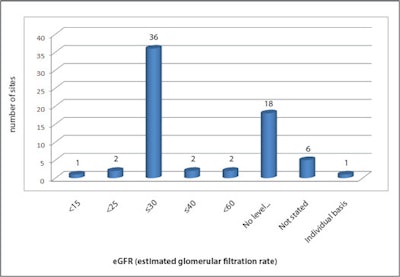 Renal function levels at which contrast is contraindicated.
Renal function levels at which contrast is contraindicated.Participants were asked to state renal function levels at which contrast administration was contraindicated (see figure). Despite guidance advising against GBCA exposure in patients with estimated glomerular filtration (eGFR) levels below 30 mL/min/1.73 m2, three sites indicated adoption of lower thresholds, and 18 sites did not identify cut-off levels for administration.
One site stated decisions were made on a case-by-case basis following risk/benefit assessment. Another specified that renal function thresholds for contrast were based on the type of GBCA used (eGFR > or equal to 60 mL/min/1.73 m2 for the moderate risk type and eGFR > or equal to 50 mL/min/1.73 m2 for low risk agents).
"Several resultant prophylactic strategies were described," added Clarke, who is a specialist in adult education, curriculum theory, and teacher education, and has an MSc degree in medical imaging. "Follow-up renal function testing is not required in any of these guidelines, yet a small number of sites are performing it."
Nephrogenic systemic fibrosis is an iatrogenic scleroderma-like systemic disorder occurring in patients with severe or end-stage renal disease -- gadolinium chelates have been identified as a causal agent, she continued. Contributory factors have been suggested, but the association has been proven in patients with impaired renal function. The recommended screening process for patients at risk varies between guidance, from assessment of renal function on all patients to risk stratification with a prescreening questionnaire and blood tests only in the high-risk group.