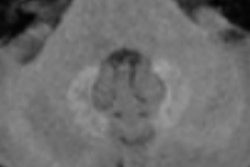
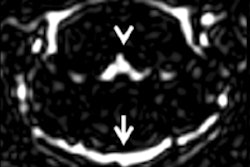
Since the peak of the nephrogenic systemic fibrosis (NSF) crisis in 2007-2008, cases have declined and are rarely, if at all, seen today.1 This is because shortly after the recognition of NSF and its connection to gadolinium-based MRI contrast agents (GBCAs),2 very effective countermeasures were drawn up and consistently implemented.
What lessons can be learned from the NSF episode?
First, "obvious" conclusions are not always correct. All new findings should be investigated thoroughly, and methodologically robust clinical evidence must be obtained before conclusions are drawn.
 Dr. Johannes Heverhagen, PhD.
Dr. Johannes Heverhagen, PhD.The initial "obvious" conclusion that macrocyclic agents are entirely safe and that linear agents are entirely culpable for NSF has been proven wrong with the demonstration of NSF cases following the sole administration of the macrocyclic agent gadobutrol (Gadovist) and the lack of NSF cases following administration of the linear agents gadobenate dimeglumine (MultiHance) and gadoxetate disodium (Primovist/Eovist). Clearly, the initial belief that the chemical structure of GBCAs was the only contributing factor to NSF was wrong.
Second, we should not draw premature or incomplete conclusions. As a consequence of the initial belief that NSF was related solely to the administration of linear agents, even linear agents without a single unconfounded case of NSF are still classified as being of intermediate risk in Europe, based solely on their chemical structure and not on the basis of clinical evidence and proper risk evaluation. This confuses many radiologists to the point they avoid the use of these agents, potentially to the detriment of the patient.
Third, we should treat imaging findings with caution in the absence of clinical correlations or symptoms. This relates mainly to the newly found hyperintensities in the brain after the application of multiple doses of GBCAs. Many people are again calling for the "dangerous" linear agents to be removed from the market. However, as yet, no clear correlations have been drawn between imaging findings and clinical outcome for any agent. All results have come from small, single center, retrospective studies that are particularly susceptible to bias.
Now, people say: "What do we lose by eliminating the linear agents? We still have the macrocyclics." However, how do we know these linear agents cause any harm? Moreover, how do we know the macrocyclics do not cause harm, and maybe cause other, yet undiscovered diseases? Why should we eliminate perfectly safe and possibly more effective drugs from the market without a need to do so? Moreover, without thorough research, the medical community is risking the removal of all MR agents from the market because recent data suggest that all GBCAs deposit gadolinium in the brain, even macrocyclics after one single injection.
Context and background to NSF crisis
The first gadolinium-based contrast agent was introduced in the late 1980s/early 1990s. Although a dose of 0.1 mmol/kg bodyweight was recommended, doses of 0.3 mmol/kg bodyweight and higher were frequently utilized, particularly for applications such as brain tumor imaging and MR angiography.
In 2003, a textbook of MR angiography stated: "From an image quality point of view, generally the more contrast the better."3 It went on to say: "Gadolinium compounds have no clinically detectable nephrotoxicity. They can be used safely at the maximum dose in patients with renal failure."
However, in 1997, Cowper and colleagues noted previously unknown fibrosing skin lesions in 15 patients.4 All the patients were undergoing dialysis due to end-stage renal disease (ESRD). The authors designated the skin lesions as "nephrogenic fibrosing dermopathy." The condition, now referred to as NSF, occurs almost exclusively among patients presenting with severe impairment of kidney function with an eGFR below 15 ml/min/1.73 m
In June 2006, the FDA issued an announcement stating high doses of gadolinium-based contrast media should be administered only in cases of absolute necessity in patients with eGFR ≤ 15 ml/min/1.73m2.7 The FDA required manufacturers to include a warning notice in the Summary of Product Characteristics (SPC) regarding patients with severe renal insufficiency early in 2007.8
In addition, other imaging procedures for patients with moderate to severe renal insufficiency were advocated. The European authorities reacted similarly, and in Germany "Red Hand" letters were issued with approval restrictions for patients with renal insufficiency (2/2007 gadodiamide9 and 6/2007 gadopentetate dimeglumine).10
Shortly after the connection between NSF and GBCAs was discovered, it became clear that certain linear agents, such as gadodiamide (Omniscan), gadoversetamide (Optimark), and gadopentetate dimeglumine (Magnevist) were more prone to association with NSF than certain macrocyclic agents, such as gadoteric acid (Dotarem), gadobutrol (Gadovist), and gadoteridol (ProHance).
Linear agents were associated with relatively high numbers of NSF cases, while macrocyclic agents had none. This seemed to reflect the greater stability of the macrocyclic agents and led to classifications in which macrocyclic agents were deemed low risk for NSF, while linear agents were labeled as "medium" or "high risk."
Subsequent findings, however, revealed further differences between the different GBCAs than just linear and macrocyclic. First of all, dose is an important factor. Almost all patients who developed NSF received single high or multiple doses of GBCAs. Inadvertent high dosing might also be the reason that some cases of NSF are now associated with the more concentrated macrocyclic agent gadobutrol (Gadovist).
Second, molecular charge also plays an important role. Nonionic linear agents are associated with substantially more NSF cases than ionic agents.
Third, the elimination route is important. Partial elimination of gadobenate dimeglumine (MultiHance) and gadoxetate disodium (Primovist/Eovist) by the liver potentially contributes to the fact no cases of NSF are known following the exclusive use of either of these agents.
Thus, despite their linear structure, these agents are not contraindicated in patients with impaired kidney function while Omniscan, Optimark, and Magnevist are. Last but not least, exposure of vulnerable patients is an important contributor. In this regard, gadobutrol (Gadovist) was not approved in the U.S. until 2011 and gadoteric acid (Dotarem) until 2013. Thus, vulnerable patients in the U.S. were not exposed to these agents at the height of the NSF crisis. Moreover, exposure to these agents in the U.S. has only occurred since the implementation of appropriate safety measures.
Dr. Johannes T. Heverhagen, PhD, is professor and head of radiology, neuroradiology, and nuclear medicine at Inselspital, University Hospital of Bern in Switzerland.
References
- Becker S, Walter S, Witzke O, et al. The German registry for nephrogenic systemic fibrosis: Findings from 23 patients. Clin Nephrol. 2010;73(6):426-430.
- Grobner T. Gadolinium -- a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
- 3D contrast enhanced angiography. 3rd ed. Berlin, New York: Springer Publishers, 2003:22-23.
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
- Jalandhara N, Arora R, Batuman V. Nephrogenic systemic fibrosis and gadolinium-containing radiological contrast agents: An update. Clin Pharmacol Ther. 2011;89(6):920-923.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
- Advisory FPH. Gadolinium-containing Contrast Agents for Magnetic Resonance Imaging (MRI). 2006.
- FDA. New warnings required on use of gadolinium-based contrast agents. 2007.
- Healthcare G. Wichtige Information zur Arzneimittelsicherheit Omniscan und nephrogener systemischer Fibrose. 2007
- Care BH. Aktualisierte Sicherheitsinformation zu Magnevist und nephrogener systemischer Fibrose (NSF). www.akdae.de/Arzneimittelsicherheit/RHB/Archiv/2007/46-20070625.pdf. 25 June 2007. Accessed 21 March 2016
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.