While new studies are examining the long-term toxicity of gadolinium chelates, the regulatory authorities are debating which measures should be adopted for regulations and pharmacovigilance. A current investigation by the European Medicines Agency (EMA) to evaluate the issue of intracerebral hypersignals and assess risk/benefits should bring concrete answers in the months to follow.
Gadolinium contrast products for MRI were considered very safe until 2007. The discovery of nephrogenic systemic fibrosis (NSF) linked to intravenous administration of gadolinium chelates led to a rethink on injection policy and the classification of chelates. The EMA established a chelates classification table in line with the risk of NSF based on chemical structure: linear chelates at high risk, substituted linear chelates at medium risk, and macrocyclic chelates at low risk. Contraindication for the intravenous administration of high-risk chelates in patients with severe renal impairment has meant this disease has now disappeared.
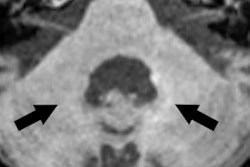
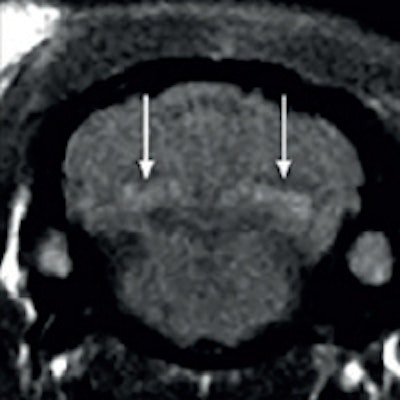
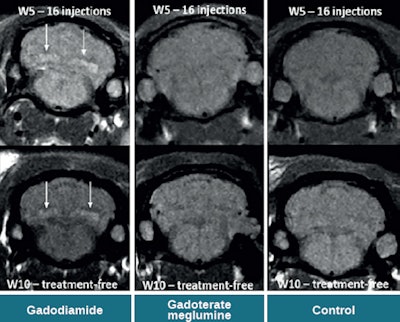 Spontaneous T1 hypersignals in a rat after repeated injections of gadodiamide and gadoterate meglumine. Figure courtesy of Dr. Pierre-Henri Lefevre, republished from le Quotidien des JFR.
Spontaneous T1 hypersignals in a rat after repeated injections of gadodiamide and gadoterate meglumine. Figure courtesy of Dr. Pierre-Henri Lefevre, republished from le Quotidien des JFR.A new series of studies concerning the intracerebral retention of gadolinium has yet again thrown into question the long-term toxicity of gadolinium chelates, in particular the linear type:
- The first article by Kanda et al1 showed T1 hypersignal of the globus pallidum and the dentate nucleus of the cerebellum in patients who had been administered several injections of gadopentetate dimeglumine or gadodiamide
- Errante et al2 then showed T1 hypersignal of the dentate nucleus in patients who had received several doses of gadodiamide
- The second article by Kanda et al3 showed an absence of hypersignal with macrocyclic gadoteridol
- Quattrochi4 confirmed T1 hypersignal after gadodiamide in patients undergoing repeated follow-up exams for meningioma, with no systemic interval therapy
- The study by Radbruch et al5 confirmed this effect is dependent on the class of gadolinium product
- Finally, two recent studies6,7 in Radiology found an accumulation of gadolinium in the brain of patients examined postmortem with mass spectroscopy
In 2016, several other animal studies complemented the data.8,9 They showed that hypersignals were reproducible in animals after large dose injections of linear gadolinium chelate (Omniscan, Magnevist, MultiHance), but not macrocyclics (Gadovist, Dotarem).
The mechanism of transfer is to the cerebral spinal fluid by the choroid plexus, then retention in the interstitial cerebral tissue of the dentate nucleus in the cerebellum. To obtain a T1 hypersignal, the gadolinium has to contain macromolecules of the interstitial tissue for relaxivity to be shown.
Hypersignal of the dentate nucleus has never been observed in the significant numbers of patients injected with macrocyclic chelates in a clinical setting. Conversely, hypersignal proportional to the number of doses of gadolinium injected has been observed with linear chelates and MultiHance. These cerebral hypersignals remain at present simply imaging observations and are not for the moment correlated to specific clinical signs, in particular, the regulation of movement controlled by the dentate nucleus. Moreover, contrary to NSF, intracerebral hypersignals are observed in patients who have normal renal function.
Regulatory aspects
The EMA has launched an investigation in line with article 31 of the European directive and has asked the Pharmacovigilance Risk Assessment Committee (PRAC) to evaluate intracerebral hypersignals and assess the risk/benefits of possible modifications to marketing authorization. The investigation is underway and, by the end of September, PRAC was due to submit a definitive report that will serve as the basis for a decision by the EMA concerning marketing authorization. Therefore in the coming months we should have a clear response from EMA on matters of toxicity and pharmacovigilance.
In conclusion, intracerebral hypersignals are observed for multiple doses through repeated administration of gadolinium chelates for MRI. At-risk patients are therefore children, patients with chronic pathologies who will have repeated MRI tests (inflammatory disease, multiple sclerosis, Crohn's disease), and populations with cancer risk factors (BRCA1 mutation for breast cancer).
Will the EMA decide only to modify the recommendations before administration for at-risk populations, or will it considerably change marketing authorization by limiting indications for at-risk populations? We'll find out in the coming months.
Additional report from Dr. Pierre-Henri Lefevre, CHU Nîmes:
Contrast agents are routine in radiological practice, but whether gadolinium- or iodine-based, they always raise the question of safety. The emergence of NSF after intravenous administration of gadolinium chelates led to the recent modification of practice guidelines. Since 2014, a new dilemma has appeared concerning gadolinium products, after the discovery of gadolinium deposits in the brain in patients undergoing multiple injections.
The JFR 2016 session "Contrast products and brain imaging" covered the interactions between iodine and gadolinium contrast products and the nervous system. A true pillar of cerebrovascular physiology, the blood-brain barrier remains impermeable to contrast products. Nevertheless, Dr. Nicolas Menjot de Champfleur from Montpellier underlined the existence of zones that present physiological enhancement after injection, such as the pituitary stalk, the pituitary gland (hypophysis), the pineal gland, and the choroid plexus.
Furthermore, the neurotoxicity induced by iodine contrast products was noted, particularly in the context of interventional neuroradiology; cases of encephalopathy induced by contrast products are rare but reported, with spontaneous resolution in the vast majority of cases. Conversely, specific neurotoxicity induced by gadolinium has not been proven in living patients, despite the abundant literature showing neurotoxicity in rats.
The question that enlivened the debate and that was clearly presented by Dr. Olivier Clément remains in play: What are the gadolinium deposits depicted in patients undergoing multiple injections?
The recent literature shows a strong correlation between the accumulated quantity of injected gadolinium and the intensity of spontaneous T1 hypersignal in the globus pallidus and the dentate nucleus. It is noteworthy this correlation is proven for linear gadolinium chelates, but not found in patients who were only administered with macrocyclic products. Histological data, however, shows intracerebral deposits of gadolinium even in patients injected with macrocyclic products. It was reiterated that to date, no clinical sign has been associated with hypersignals.
The issue of practice therefore principally concerns patients who must undergo follow-up by MRI that necessitates repeated injections. Multiple sclerosis (MS) carriers, a cohort in whom hypersignals have been observed, pose a problem in routine practice for the radiologist: Should I still inject MS patients?
With this opening question, Dr. Thomas Tourdias (Bordeaux) noted today contrast injections in MS cases are unavoidable because they are crucial to therapeutic management. Research is underway, notably in parametric imaging to allow for the absence of intravenous administration, but for the moment there is no reliable alternative in clinical practice. The advantage of this developing debate has been to revitalize a medical dogma that is fundamental to imaging: primum non nocere -- first, do no harm.
Dr. Olivier Clément, PhD, is a radiology professor at Georges Pompidou European Hospital in Paris and Dr. Pierre-Henri Lefevre is a neuroradiologist at CHU Nîmes.
Editor's note: This is an edited translation of two articles published in French on 14 and 15 October 2016 by le Quotidien des JFR (Journées Francophones de Radiologie), the daily newspaper of the JFR. Translations by Frances Rylands-Monk.
References
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841.
- Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014;49(10):68590.
- Kanda T, Osawa M, Oba H, et al. High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology. 2015;275(3):803-809.
- Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, et al. Gadodiamide and Dentate Nucleus T1 Hyperintensity in Patients With Meningioma Evaluated by Multiple Follow-Up Contrast-Enhanced Magnetic Resonance Examinations With No Systemic Interval Therapy. Invest Radiol. 2015;50(7):470-472.
- Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015;275(3):783-791.
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015;275(3):772-782.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology. 2015:142690.
- Robert P, Violas X, Grand S, et al. Linear Gadolinium Based Contrast Agents Are Associated With Brain Gadolinium Retention in Healthy Rats. Invest Radiol. 2016;51(2):73-82.
- Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H. Signal Increase on Unenhanced T1-Weighted Images in the Rat Brain After Repeated, Extended Doses of Gadolinium-Based Contrast Agents: Comparison of Linear and Macrocyclic Agents. Invest Radiol. 2016;51(2):83-89.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.