An evaluation of two linear gadolinium-based contrast agents (GBCAs) showed no signs of abnormalities in the brains of rats despite the presence of gadolinium. What's more, researchers found that gadolinium levels had decreased markedly 20 weeks after GBCA injection, according to results published online September 27 in Radiology.
The encouraging findings come from researchers at GE Healthcare who measured miniscule amounts of gadolinium in the rats' brains after multiple doses of gadodiamide (Omniscan, GE) or gadopentetate dimeglumine (Magnevist, Bayer HealthCare). They also noted no residual histopathologic or adverse effects associated with the GBCAs (Radiology, September 27, 2016).
 Dr. Mark Hibberd from GE.
Dr. Mark Hibberd from GE."It is important to remember that the doses given to rats were well above those that would ever be given to a [human] patient within a much shorter time frame," said study co-author Dr. Mark Hibberd, head of global medical services and chief medical officer for life sciences at GE. "It is also important to emphasize that gadolinium present in the brain at these very low levels has not been shown to have harmful short- or long-term effects."
Gadolinium research
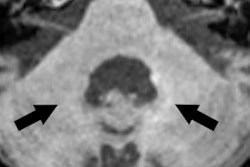
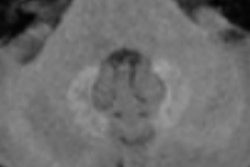
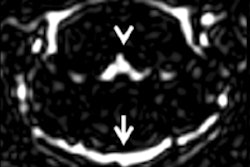
The recent controversy over gadolinium retention in humans began almost three years ago with a study by Kanda et al. The group found high signal intensity on unenhanced T1-weighted MRI scans in the dentate nucleus and globus pallidus of the brain in 19 patients who had received six or more GBCA injections -- long after the injections took place.
More recently, a 2016 study by Prince et al also discovered higher levels of signal intensity on unenhanced MR images of the brain among patients who received more than 35 injections of gadodiamide, gadobenate dimeglumine, or gadopentetate dimeglumine. One key finding was that the phenomenon appeared to have no clinical effect on patients.
Animal studies also have been performed, with French researchers Robert et al finding increased deposits of gadodiamide, which over time produced "significant and persistent" MRI abnormalities in the brains of rats. By comparison, no such T1-weighted hyperintensities were evident in the brains of rats injected with gadoterate meglumine or a saline solution.
But how fast does gadolinium clear from the brain? That's a question that has not been explored in the studies on humans, the GE researchers wrote.
"While these studies demonstrated an association between the amount of GBCA administered and the amount present in the brain tissues of deceased patients, the investigators did not explore the pharmacokinetics of gadolinium presence in the brain, leaving open the question of the potential time dependence of observed gadolinium concentration," they wrote.
Dosage protocols
In their study, six rats each were randomly assigned to seven treatment groups and given a linear GBCA at a daily dose of 0.6 mmol per kilogram of body weight. That amount is equivalent to a typical human dose of 0.1 mmol per kilogram of body weight, after adjusting for body surface area.
"Rats are widely used in nonclinical development of pharmaceutical products during testing for effectiveness and safety," Hibberd wrote in an email to AuntMinnie.com. "Many years of experience have demonstrated the relevance of these models for assuring safety before advancing to human studies, and they are accepted by regulatory authorities and scientists around the world."
Gadodiamide or gadopentetate dimeglumine was administered intravenously. Rats in the low-dosage groups received two doses per week for five weeks, while the high-dosage groups received four doses per week for five weeks. There was also a control group given a saline solution.
"With these regimens, we attempted to model potential repeat total cumulative dose exposure that might occur in the clinic, albeit over a period of five weeks in this study, compared with a period of months to years in a realistic clinical time frame," the authors wrote.
The researchers then measured gadolinium levels in the blood and brains of rats one week after injections and 20 weeks later with up to 20 repeat doses of GBCA (cumulative dose, 12 mmol per kilogram of body weight).
Gadolinium retention
In the follow-up analysis, the researchers detected a maximum level of gadolinium in the rats' brains equal to 0.00026% of the injected dose one week after injections. After 20 weeks, gadolinium levels decreased to 1/1,000,000th of the injected cumulative dose.
Levels of gadolinium one week after dosing (2.49 nmol per gram of brain tissue, or 0.00019% of the injected dose) decreased by approximately 50% (to 1.38 nmol per gram, or 0.00011% of the injected dose) some 20 weeks later.
Most importantly, the researchers noted no histopathologic or adverse findings associated with the levels of gadolinium, nor any evidence or neurotoxicity from the GBCAs.
The results are applicable to human patients for several reasons, Hibberd noted. First, animal investigations are considered valuable surrogates for clinical studies in situations where controlled clinical trials are not feasible or ethical. Second, rat model studies are commonly used when drugs are developed or when further assessments of safety are required.
"Third, the clinical data available are consistent with the findings from the rat studies, as there are neither adverse histological changes in the rat brain nor adverse clinical effects that have been identified in patients," he said. "Furthermore, the levels of gadolinium observed in the rat brain are within the range of values seen in published human autopsy studies."
Additional research
The GE group plans to advance the research in several ways. Initially, they will assess the potential for longer-term effects in the brain with one-year follow-up in rats.
"Second, we are conducting ultrastructural studies that will examine the brain for details of cell morphology at higher resolution than standard histopathology," Hibberd said. "Third, we have collaborations with independent scientists to examine how different GBCAs compare with each other, and the distribution and chemical form of gadolinium in the brain."
In addition, as research continues on GBCAs, GE plans to collaborate with the U.S. Food and Drug Administration, the American College of Radiology, the clinical community, academics, and regulators to gather evidence on any adverse consequences of accumulation of gadolinium in the brain.