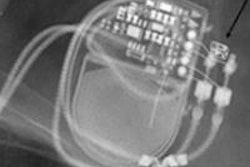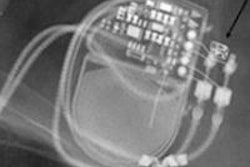
Third-generation dual-source CT scanners can now provide fast, comprehensive, and complementary information in the evaluation of mechanical heart valves, award-winning Dutch researchers reported at last month's RSNA meeting in Chicago.
Although the radiation dose is higher than in modern CT of the coronaries due to the dynamic character and higher kV levels of the scan, the severity of the problems and critically ill condition of patients justifies the use of CT as a diagnostic tool in the evaluation of mechanical heart valves, according to lead author Marcel Dijkshoorn, a CT radiographer at the Erasmus Medical Center in Rotterdam.
"Dual-energy CT has the potential to further eliminate metal artifacts by reconstructing monoenergetic keV metal artifact reduction datasets," he noted in an e-poster that received a certificate of merit. "Dual energy, however, has some drawbacks: temporal resolution is lower, making the scan more vulnerable to motion and less suitable for high heart rates; the high keV not only reduces metal artifacts but also contrast enhancement (up to 85%); and mechanical evaluation is better but visualizing leakage pannus or thrombus is very difficult."
Competing techniques
Transthoracic and transesophageal echocardiography and fluoroscopy are used to evaluate suspected mechanical valve dysfunction, but they may not allow determination of the cause of dysfunction. CT provides additional information and is a promising, complementary technique, especially in patients with obstruction and endocarditis (see table).
| Diagnostic imaging tools | |||
| Ultrasound | Fluoroscopy | CT | |
| Valve dynamics | Good | Good | Good |
| Thrombus/pannus | Moderate | No | Good |
| (Para)valvular leakage | Yes | No | Limited (no flow) |
| Infection/abscess | Moderate | No | Good |
| Artifacts | Acoustic shadowing | No | Beam hardening and motion |
| Contrast | No | No | Yes |
| Radiation | No | Yes | Yes |
| Patient burden | TTE low - TEE high | Low | Low |
| Costs | TEE low - TEE high | Low | Medium |
Over the past 50 years, more than 80 models of prosthetic heart valves have been developed. The mechanical heart valves can be divided into these main types: ball-in-cage valves (older type still seen in patients but rarely implanted today), tilting disk valves, and bileaflet valves (most commonly used).
An important potential problem with heart valves is regurgitation, where the valve does not fully close and part of the blood flows back into the heart chamber, the authors pointed out. Potential causes are congenital bicuspid valve; rheumatic disease; endocarditis; calcific degeneration; collagen disorders (e.g., Marfan syndrome); sustained, severe, high blood pressure; ischemic heart disease with mitral annulus dilatation; and aortitis, ascending aortic aneurysm and aortic dissection. The effect is volume and pressure overload, which over time can lead to heart chamber dilatation and congestive heart failure.
Another issue is stenosis. Because the valve does not fully open, the heart has to pump harder to get sufficient blood flow through the valve. The causes may be valve thickening or stiffening by degenerative "wear and tear" or severe calcification buildup, often related to diabetes mellitus, hypertension, and/or hypercholesterolemia. Other causes are congenital valve fusion and rheumatic fever. As a result, there is a risk of bacterial endocarditis, angina, syncope, ventricle hypertrophy, and congestive heart failure.
"An important cause of valve obstruction is formation of thrombus or pannus tissue. Pannus tissue is a form of fibrous scar tissue," Dijkshoorn noted. "Complications due to thrombus are usually (sub)acute events, while complaints due to pannus are often more chronic and slow developing."
Rotterdam's CT protocols
The authors use the following scan protocol setup for the evaluation of mechanical valves: native prospective triggered scan (scan range limited to cover the only the valve, minimized ECG padding set to systole); arterial prospective triggered scan, a fixed scan range of 14 cm centered around the valve, kV modulation switched to minimal semi 120 kV; dynamic multiphasic reconstruction at 5% RR ECG intervals, venous high pitch prospective spiral TurboFlash scan; scan range full thoracic aorta, able to show extent of disease not limited to the valve; and better phase for depicting thrombus and differentiate thrombus from flow artifact.
"Although retrospective gating is commonly used for dynamic scans we prefer to use prospective triggering," they wrote. "Why choose prospective triggering? High pitch spiral scan mode, it will not give stacks but does not provide dynamic information. Retrospective gating scan mode: because of oversampling by low pitch value, more stacks are needed for the same scan length and the location of the stacks are uncertain. Radiation dose will also increase."
With prospective protocols in third-generation dual-source CT, the collimation is adapted to the set scan length, and in regular cardiac scans, this helps to avoid overscanning and results in an interpatient variability of the stack width, the authors added.
Furthermore, when scanning valves, full heart coverage is not necessary and a shorter range is sufficient. Special care should be taken in planning the scan range: a short range consisting of two stacks will result in potential stack artifacts through the valve, and a longer scan range will result in three, thin collimated stacks, with the valve in the middle of the stack. Heart motion and respiratory variability might still move the valve into the potential stack artifacts, while opening the scan length to exactly 14 cm will result in three stacks with maximum stack width, ensuring valve images without ECG stack artifacts.
For dynamic CT angiography, the team prefers an ECG trigger based on absolute millisecond (msec) time instead of a relative (%) RR trigger. A "scan on-scan off" padding setting from 0-1,500 msec with 50-500 msec ECG pulsing results in a scan acquisition window that is guaranteed to provide the fastest onset of radiation after an R peak (minimum delay is ± 100 msec, which is 10% at 60 beats per minute [bpm]). Radiation is maintained for 1,500 msec, which is a full heart cycle at 40 bpm, or is cut off when the next R peak occurs. This means that independent of the heart rate -- no matter if it is high, low, variable, or irregular -- a full heart cycle starting from 100 msec is guaranteed. This makes a very robust and easy protocol, and there is no need to think about the heart rate, according to Dijkshoorn and colleagues.
"With the selection of kilovolt peak, a trade-off has to be made between beam-hardening artifacts, which are reduced at higher kVp; radiation dose, which can be decreased at lower kVp in contrast-enhanced scans; and contrast enhancement increases at lower kV, and the improved enhancement can be used for quality gain or reduction of the required total contrast material volume," they stated.
Imaging of the Bjork-Shiley valve can benefit from selecting 150 kVp because the dense metal components cause severe beam-hardening artifacts, and this will result in a higher average radiation dose for these patients. To further minimize artifacts, the window width/center should be increased, and injecting a stronger contrast bolus can compensate contrast density loss in the image.
On contrast administration, the authors made the following points:
- Image quality benefits from a contrast bolus with a high iodide delivery rate of ± 2.0 grams of iodine/sec.
- A high-contrast attenuation facilitates wide window settings, which helps reduce the severity of artifacts.
- CT angiography scan time is short, which should allow short bolus with a low volume of contrast, but the need for sufficient venous enhancement requires a higher volume of ± 80 mL.
- The vast majority of mechanical valves are in mitral or aortic position. Be aware of and adapt timing to tricuspid or pulmonary valve positions.