MRI examinations of patients with pacemakers can be performed safely when proper device programming and monitoring are adhered to, and there do not appear to be major differences between the results of MR-conditional and MR-unsafe devices and between scans of the thorax and of other scanning regions, Finnish researchers have found.
"Radiologists should plan scanning protocols, and a physicist should assist in adjusting scanning parameters in order to ensure sufficient image quality for diagnosis," noted Touko Kaasalainen, a medical physicist at HUS Medical Imaging Center, and colleagues from Helsinki University Central Hospital. "Additionally, MRI radiographers should be familiar with the safety procedures for scanning these patients and also be prepared to immediately remove the patient from the MRI scanner upon discovery of a significant problem. Furthermore, proper resuscitation equipment should be at hand and ready for immediate use in case of an emergency."
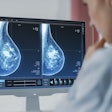
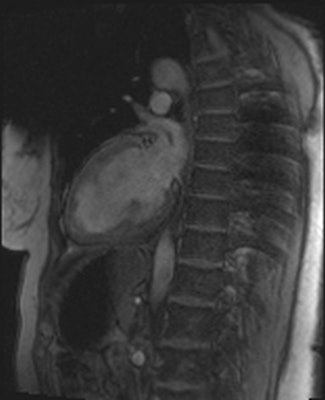 Artifacts are shown in cardiovascular MR patient imaged using spoiled gradient echo cine sequences in short-axis and two-chamber planes. Images courtesy of Touko Kaasalainen.
Artifacts are shown in cardiovascular MR patient imaged using spoiled gradient echo cine sequences in short-axis and two-chamber planes. Images courtesy of Touko Kaasalainen.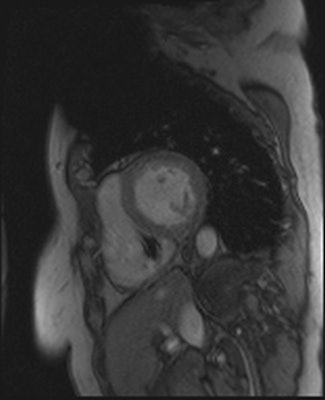
Patient evaluation and safety decisions must be done by consultation between the radiologist and cardiologist. Also, because deciding on the appropriate pacing mode during the MRI examination requires arrhythmia expertise -- as well as an understanding of the patient's initial indication for the device, arrhythmia history, and underlying rhythm/pacemaker dependency -- a cardiologist is needed to perform pacemaker interrogations and programming before and after MRI, they explained in an article-in-press published online by the European Journal of Radiology (EJR) on 1 May 2014.
A cardiologist or radiologist should be present to monitor the patient during MRI, and because radiofrequency and gradient pulses sometimes strongly affect ECG, pulse oximetry is also necessary to provide a reliable monitoring signal.
Staff at the Helsinki facility has now scanned around 190 patients, including those with implanted cardiac pacemakers (PMs), implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices, and only a few changes in pacing rate have occurred when patients undergo MRI. Cardiologists do not participate in patient monitoring during MRI, and this is done by radiologists.
More than two million patients worldwide have one of these devices, and estimates indicate that patients with a PM or ICD have a 50% to 75% likelihood of showing a clinical indication for MRI over the lifetime of their cardiac device, according to Kaasalainen, who is currently working on his PhD dissertation, due for completion this year.
Because of the increasing prevalence of cardiac pacing systems and the high frequency of clinical indications and needs for MRI, manufacturers have introduced MR-conditional PM and ICD systems that permit safe MRI examinations under certain conditions. Also, despite the potential for adverse outcomes, patients with MR-unsafe PMs and ICDs have been scanned using particular precautions.
The guidelines of the European Society of Cardiology (ESC) state that patients with a selected modern cardiac device can be scanned with an acceptable risk/benefit ratio, even though the cardiac device bears no MR-conditional labels, the authors stated.
The aim of their EJR study was to introduce and evaluate safety protocols for performing MRI examinations of patients with different cardiac pacing devices. Between November 2011 and May 2013, they performed 68 consecutive examinations on 64 patients with pacing devices, using a Magnetom Avanto 1.5-tesla MRI scanner (Siemens Healthcare) with a maximum gradient field of 45 mT/m and a slew rate of 200 T/m/s.
Of the total, 60 patients (94%) had a PM, including 22 (37%) MR-conditional, and 38 (63%) MR-unsafe PMs, while two patients (3%) had an MR-unsafe CRT device and two (3%) had an MR-unsafe ICD system. Four patients (6%) were scanned twice (two with an MR-unsafe PM and two patients with an MR-conditional PM), and six patients had two body regions scanned in the same MRI examination.
The mean age of the patients was 67 ± 14 years, and 42% (27/64) were women. Altogether 21 (31%) of the examinations were scans of the thorax area, and 20 (29%), 17 (25%), and 16 (24%) of the examinations were MRI scans of spine, head, and heart respectively. The remainder was scans of the pelvis, liver, vagina, rectum, wrist, lung, carotid artery, and soft tissue of the neck, pancreas, and knee.
All MRI examinations were completed safely, reported the authors. Two patients with an MR-unsafe PM (Guidant Insignia I Entra SR and Medtronic Kappa KSR 401) experienced a change in pacing rate. They observed no unexpected changes in the heart rate or rhythm, nor did they see any shock deliveries, sustained atrial, or ventricular arrhythmias during the MRI examinations. None of the patients reported any torque or heating sensations, palpitations, pain, dizziness, or other unusual symptoms during MRI.
"We saw no evidence of device malfunction either immediately after MRI scans or during repeat testing one month after examination. We were able to interrogate each pacing device normally after MRI, and found no changes in the programmed parameters," they noted.
The study, which was supported by the State Subsidy for University Hospitals in Finland, had some limitations, the authors conceded. The sample size was relatively small, and only two patients had a CRT system and two others had an ICD device. Also, the study had a limited number of cardiac pacing device models to study, and several cardiologists programmed and measured the devices prior to and after MRI, which may have caused variations in the measurements.
Finally, it's unclear if one month follow-up was sufficient to assess the consequences of lead-tip heating, and the present findings are limited to 1.5-tesla machines.
Study disclosures
One of the authors (Sami Pakarinen, HUS Department of Cardiology, Helsinki University Central Hospital) declared a conflict of interest, having been a consultant for St. Jude Medical, Medtronic, Boston Scientific and Biotronik.
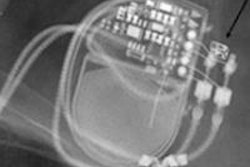
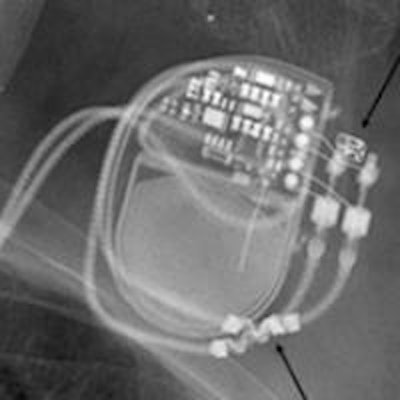
Editor's note: The image on our home page shows a pre-MRI x-ray scan, which allows visual confirmation of electrode placement and integrity, implantable pulse generator position, and MR-conditional radiopaque markers. Image courtesy of Dr. Josef Vymazal, head of radiology at Na Homolce Hospital, Prague.