Prompted by apparent gaps in a 2013 report from the European Society of Cardiology (ESC), the German Radiological Society (DRG) has issued its own guidelines on MRI exams of pacemaker patients. Radiologists must be familiar with the current advice and work closely with cardiologists, says the DRG.
To shed light on the latest thinking about pacemakers and MRI, Dr. Matthias Gutberlet, a professor in the Leipzig University Cardiology Centre and chairman of the Cardiovascular Diagnosis Working Group of the DRG, shares his views in an interview.
 Radiologists must know all about pacemakers to take the essential decisions, says Dr. Matthias Gutberlet.
Radiologists must know all about pacemakers to take the essential decisions, says Dr. Matthias Gutberlet.
Why has the Cardiovascular Diagnosis Working Group published a position paper on MRI examinations on patients with pacemakers?
Gutberlet: Two years ago the European Society of Cardiology (ESC) published a position paper called "MRI scans on patients with pacemakers," but, in our opinion, it did not fully cover this subject, which remains a complex one.
It was very commendable that the ESC paper noted there is normally no longer an absolute contraindication to MRI scans for patients with pacemakers. This means that patients with pacemakers, of whom there are probably more than 600,000 in Germany alone, are no longer automatically excluded from this valuable procedure. However, the impression was given that practically any patient with a pacemaker that has a safety rating of "MR-conditional" could undergo MRI scanning without any problems, provided that the cardiologist has correctly reprogramed the pacemaker beforehand.
What can happen if a patient with an MR-conditional pacemaker has an MRI scan?
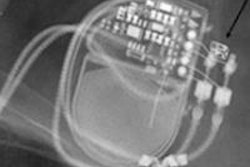
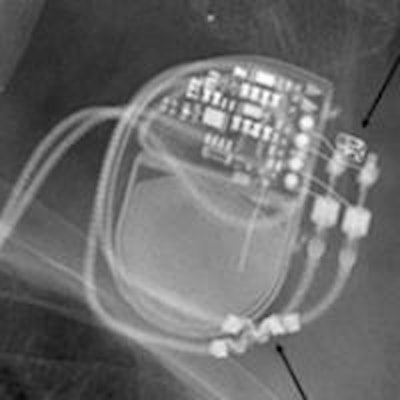
Unfortunately, an MRI scan performed on patients with an MR-conditional pacemaker can constitute off-label use and may even be dangerous for the patient. This is the case if the manufacturer's instructions for use are not followed to the letter. The radiologist conducting the examination is primarily responsible. For instance, some pacemakers are approved for full-body MRI scans, while others explicitly leave out MRI scans of the heart and chest area. This has understandably led to some irritation, both for patients and healthcare professionals, particularly because pacemakers that are currently available and are not MR-conditional can be used in MRI scans under certain conditions.
Is it ever possible to give very clear, generally valid guidance, even though the issue of MRI scans on patients with pacemakers is such a complex one?
The situation is certainly a bit more complicated than we would like, but not so complicated that it is impossible to give some general advice. But, unfortunately, it cannot be set out in a single flowchart. In addition, any radiologist who is examining patients fitted with pacemakers must always consult the manufacturers' websites referred to in the position paper to see if there are any new developments. The position paper only reflects the situation pertaining at the time it was written, so radiologists must take a more detailed look at the issue and each patient. Excellent interdisciplinary cooperation is also vital for the radiologist and the cardiologist who sets the pacemaker to the correct mode before the MRI scan and checks it again after the examination.
So good interdisciplinary cooperation is the cornerstone for MRI examinations on patients with pacemakers?
Exactly. Unfortunately, it is not as simple as having the cardiologist reprogram the MR‑conditional pacemaker to an "MR protection mode" without consulting anyone else and then the radiologist just going ahead and performing the examination. The radiologist has to ask a lot of questions, conduct some tests, and especially look closely at the indications. After all, the radiologist is responsible for deciding whether the patient with a pacemaker can undergo the examination.
We wanted to help our colleagues in reaching this decision. Our position paper makes things clearer and contains very clear directions that allow radiologists to take legally secure decisions about what examinations their patients can have and how they should be carried out. So it is worth investing half an hour in reading the position paper.
What exactly does the radiologist who is to conduct the examination need to know beforehand?
The radiologist needs to know exactly what kind of pacemaker the patient has had fitted. The patient's pacemaker card or a copy of it should be available when the patient is registered for an MRI scan and later on when he comes in for the procedure. Simply stating that the pacemaker is MR-conditional is not sufficient, because pacemakers made by various manufacturers allow or exclude a wide range of indications. So the radiologist has to be absolutely sure that the conditions for use specific to the manufacturer are met. It may well be that, even in the case of an MR-conditional pacemaker that is only approved for part of the body, it will be necessary to take the same precautions and weigh the risks and benefits in the same way as in the case of a conventional pacemaker. And as this differs for many manufacturers, a bit more digging is required.
Will the position paper be updated regularly?
In this paper, we look at the types of pacemaker that are currently on the market. As companies launch a new type almost every year, we will have to update our review regularly. We will be working with the manufacturers to try to offer this update as a service to all radiologists who are members of the DRG, probably from next year. The position paper also contains all manufacturers' contact details.
Another joint position paper is currently being prepared by cardiologists who are members of the German Cardiac Society and the Cardiovascular Diagnosis Working Group of the DRG. It will give guidance on other implants, such as implantable cardioverter-defibrillators (ICDs), event recorders, and so on, but this paper probably will not be published until next year.
Editor's note: This is an edited version of a translation of an article published in German online by the German Radiological Society (DRG, Deutsche Röntgengesellschaft) in collaboration with the German Society for Neuroradiology (Deutsche Gesellschaft für Neuroradiologie). Translation by Syntacta Translation & Interpreting. To read the original article, visit the DRG website.