Digital tomosynthesis can improve the diagnostic accuracy and confidence of chest radiography in oncologic patients with suspected pulmonary lesions, and it allows CT to be skipped in about 50% of cases, Italian researchers found in a study published online on 1 December by European Radiology (ER).
Only a slight, though significant, increase in radiation dose occurred, according to Dr. Emilio Quaia, associate professor of radiology, and colleagues from the Department of Medical, Surgical and Health Sciences, Cattinara Hospital in Trieste. Their new study has not led to any changes in practice at the hospital, and the preferred strategy is to perform tomosynthesis on every patient with a suspected lesion of the chest. To improve detection of lung lesions, they now include a dual-energy technique in every oncology patient.
The authors' aim was to assess the diagnostic impact of digital tomosynthesis in oncologic patients with suspected pulmonary lesions on chest radiography. A total of 237 patients (135 male, 102 female; age 70.8 ± 10.4 years) with a known primary malignancy and suspected pulmonary lesion(s) on chest x-ray and who underwent tomosynthesis were identified. All patients revealed suspected pulmonary lesion(s) appearing as areas of increased opacity or pulmonary nodules on chest x-ray, and they underwent tomosynthesis from June 2007 to June 2014.
Two radiologists (with 10 and 15 years' experience) analyzed in consensus chest x-ray and tomosynthesis images and proposed a diagnosis according to a confidence score: 1 or 2 = definitely or probably benign pulmonary or extrapulmonary lesion, or pseudolesion; 3 = indeterminate; 4 or 5 = probably or definitely pulmonary lesion.
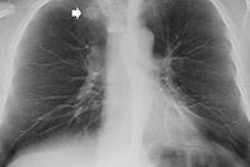
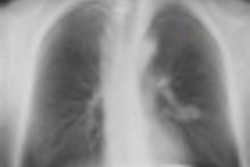
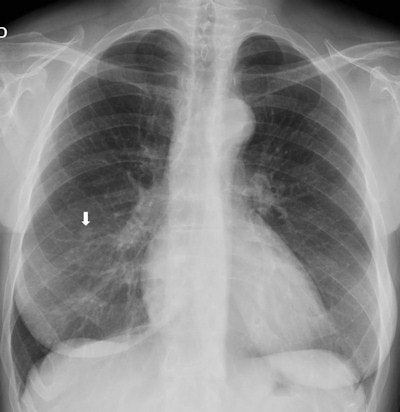
 Top: Follow-up imaging of a 72-year-old woman. Preoperative x-ray for skin melanoma. Posteroanterior chest radiography in the upright position shows one suspected pulmonary nodule in the right lung (arrow). Bottom: Digital tomosynthesis image clarifies that the same opacity corresponds to a focal sclerosis within the poster arch of a right rib (arrow). Readers provided a confidence score of 1.
Top: Follow-up imaging of a 72-year-old woman. Preoperative x-ray for skin melanoma. Posteroanterior chest radiography in the upright position shows one suspected pulmonary nodule in the right lung (arrow). Bottom: Digital tomosynthesis image clarifies that the same opacity corresponds to a focal sclerosis within the poster arch of a right rib (arrow). Readers provided a confidence score of 1.Tomography findings were proven by CT (114 patients), chest x-ray during follow-up (105), or histology (18). The final diagnoses included 77 pulmonary opacities, 26 pulmonary scars, 12 pleural lesions, and 122 pulmonary pseudolesions. Tomosynthesis versus chest x-ray presented a higher (p < 0.05) sensitivity (92% vs 15%), specificity (91% vs 9%), overall accuracy (92% vs 12%), and diagnostic confidence (area under ROC, 0.997 vs 0.619). Mean effective dose of chest x-ray vs tomosynthesis was 0.06 vs 0.107 mSv (p < 0.05).
Equipment matters
Chest x-ray examinations were obtained with a computed radiography (DirectView CR 975, Carestream) or digital radiography (Definium 8000, GE Healthcare) system. X-ray images were acquired in upright position at the wall stand with a focal spot size of 0.6 mm and a stationary antiscatter grid (70 lines per cm; ratio 13:1).
The digital radiography system with tomosynthesis capability (Definium 8000) consisted of an x-ray tube (focal spot size 0.6 mm), a wall stand, a stationary antiscatter grid (70 lines per cm; ratio 13:1), and a cesium iodide-amorphous silicon (CsI/a-Si) indirect flat-panel detector (41 x 41 cm2; 200 x 200 µm2 pixel size) with CsI in a columnar structure.
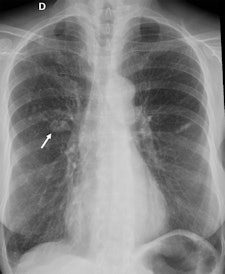
Overall, these findings are not surprising, Quaia noted, because they are similar to those in the group's 2012 publication, which included not only oncology patients but also external patients.
The principal limitation of the study was its retrospective nature, he stated. The second limitation was the consensual analysis of the chest x-ray and tomosynthesis images without assessment of the interreader variability. The third limitation was the presence of multiple reference standards including chest x-ray for pseudolesions or overt benign lesions, and CT or histology for positive pulmonary lesions.
The positive results of this study suggest that a prospective clinical trial using tomosynthesis for the management of oncologic patients is warranted, Quaia et al added.
Looking to the future, the researchers have started a phantom study with digital tomosynthesis to assess the capability of the technique to identify ground-glass nodules. They would like to investigate the results obtained by lowering the kV of the x-ray tube from 120 kV to 100 kV and 80 kV.