Cardiac arrhythmias -- abnormal heart rhythms -- can be treated by destroying dysfunctional areas of heart tissue with radiofrequency catheter ablation. But without an accurate method available to assess heat deposition and lesion extent, the success rate of this approach is only 60% when treating atrial fibrillation (the most common arrhythmia). For ventricular tachycardia, a lower success rate of just 35% has been reported.
An alternative is to use high-intensity focused ultrasound (HIFU) to ablate the cardiac tissue. HIFU can be performed through the esophagus using an endoscope, avoiding the need for catheter insertion into the heart. But here again, accurate guidance is needed to control the ablation process and monitor creation of the thermal lesion at the targeted tissue.
With this in mind, researchers from France have designed a transesophageal device that can both deliver HIFU and perform imaging. While MRI is the gold standard for HIFU guidance, it is highly challenging to perform on the heart. Instead, the researchers use cardiac shear-wave elastography (SWE) to quantitatively assess the ablation procedure.
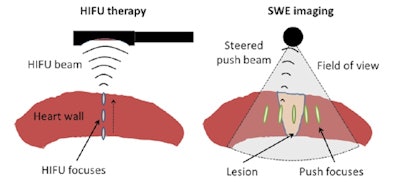 Schematic representation of the in vitro therapy-imaging configuration. Left: HIFU is performed through the entire wall depth using the electronic steering along the central axis of the probe. Right: SWE imaging is realized before and after ablation. Five to seven laterally steered pushes (focusing using the imaging probe) are performed to cover the whole field-of-view with shear-waves. All images courtesy of Wojciech Kwiecinski.
Schematic representation of the in vitro therapy-imaging configuration. Left: HIFU is performed through the entire wall depth using the electronic steering along the central axis of the probe. Right: SWE imaging is realized before and after ablation. Five to seven laterally steered pushes (focusing using the imaging probe) are performed to cover the whole field-of-view with shear-waves. All images courtesy of Wojciech Kwiecinski.SWE works by using focused ultrasound to create an acoustic force, and imaging the propagation of the resulting shear wave. The tissue stiffness is then derived from the shear-wave propagation. Importantly, it has been shown in vivo that stiffening of thermally ablated tissue is related to the applied thermal dose (Physics in Medicine and Biology, 25 September 2015, Vol. 60, pp. 7829-7846).
"Advantages of ultrasound imaging over MRI include real-time capability, to continuously provide images at the center of the treated tissue," explained Wojciech Kwiecinski, a PhD student at Institut Langevin in Paris. "SWE, which is already CE Marked and FDA approved for other applications, is not affected by cardiac motion, as stiffness maps are acquired within a single heartbeat. Ultrasound imaging is also significantly less expensive [than MRI]."
In vivo imaging
Kwiecinski and colleagues carried out an in vivo study to demonstrate that cardiac SWE can map myocardial stiffness in a beating heart. They used a standard transesophageal echocardiography (TEE) probe, comprising a 5-MHz, 64-element phased array, to perform SWE on the heart of an anaesthetized sheep in an open-chest setup.
SWE measurements recorded over a cardiac cycle showed tissue stiffness varied from 0.5 ± 0.1 to 6.0 ± 0.3 kPa in the atrium and from 1.3 ± 0.3 to 13.5 ± 9.1 kPa in the ventricles during the cycle. These values agree with previous in vivo studies on a beating heart.
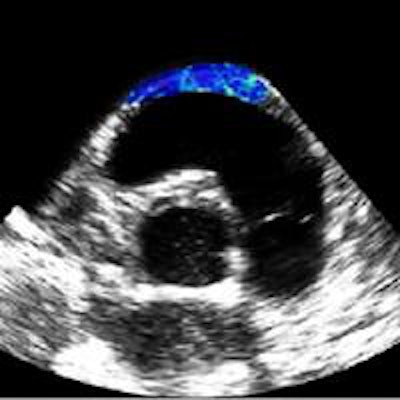
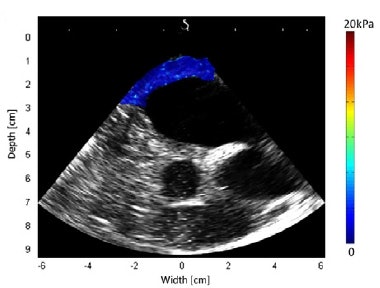 Shear modulus maps of a right atrium (above) and right ventricle free wall (below) superimposed on B-mode images. The mean shear modulus within the atrial wall, acquired at atrial systole, was 1.3 ± 0.3 kPa; the mean shear modulus in the right ventricle, acquired at ventricular diastole, was 1.7 ± 0.5 kPa.
Shear modulus maps of a right atrium (above) and right ventricle free wall (below) superimposed on B-mode images. The mean shear modulus within the atrial wall, acquired at atrial systole, was 1.3 ± 0.3 kPa; the mean shear modulus in the right ventricle, acquired at ventricular diastole, was 1.7 ± 0.5 kPa.Next, the researchers used the combined imaging-therapy endoscope (developed by collaborators from LabTau in Lyon) to demonstrate TEE-SWE mapping of HIFU-induced lesions in vitro. The combined transesophageal probe included a 3-MHz HIFU transducer, with a focal depth of 15-55 mm, with the standard TEE probe inserted at its center.
They performed HIFU and SWE on chicken breast samples and two ex vivo porcine hearts. For the chicken breast, HIFU was composed of 25 exposures with an acoustic intensity of 9 W/cm2 and a focal depth of 40 mm. In the heart, HIFU sequences (two 11 W/cm2 exposures) were performed at depths from 35 to 25 mm.
Before and after ablation, the researchers used the probe to record a B-mode ultrasound image and perform SWE mapping. Shear modulus maps recorded before and after HIFU ablation showed clear changes, with thermal lesions clearly seen on all postablation stiffness maps. In contrast, B-mode ultrasound imaging was unable to visualize the thermal lesions.
"SWE provides reliable and precise ablation extension maps," Kwiecinski said. "The link between increased tissue stiffness and thermal coagulation, combined with a high specificity, has been shown. B-mode ultrasound only provides images with very poor specificity and poor contrast."
In an example ablated zone in the chicken breast, the mean shear modulus increased from 4.8 ± 1.1 kPa before ablation to 20.5 ± 10.0 kPa after treatment. In the left atrium, the mean shear modulus increased from 12.2 ± 4.3 to 30.3 ± 10.3 after ablation. In one of the left ventricle ablations, the researchers inserted an ex vivo esophagus around the transducers and performed HIFU and imaging through the esophagus. Here, the mean stiffness increased from 21.2 ± 3.3 to 73.8 ± 13.9 kPa in the central region.
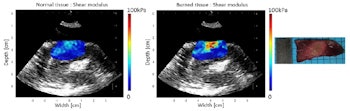
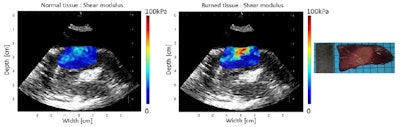
Shear modulus maps of the left ventricle, before (left) and after (center) ablation, with SWE and HIFU performed through an esophagus. The mean shear modulus increased from 21.2 ± 3.3 kPa to 73.8 ± 13.9 kPa in the central-8-mm-diameter zone. A photograph of the gross pathology section is shown on the right.
As initial tissue stiffness varied significantly between samples, the researchers calculated the ratio of shear modulus before and after ablation, setting a ratio of 1.7 (based on previous work) as a threshold to discriminate ablated and normal tissue. The mean ratios calculated for three chicken breast ablations, two ventricular ablations (one through the esophagus), and two atrial ablations were approximately 3.8, 3.0, and 2.2, respectively.
Finally, the team calculated the SWE-defined areas of the ablated lesions and compared these with areas obtained using pathology. The dimensions of the lesions measured using the two techniques agreed well.
The next stage of this study will be to induce and evaluate thermal lesions on beating hearts in vivo. "Both transesophageal therapy and imaging approaches have to be validated on a bipedal animal model. Such studies are challenging because the animal model is uncommon," Kwiecinski told medicalphysicsweb.
Kwiecinski noted that transfer to the clinic could prove a long process."The main challenge is validation of the transesophageal HIFU approach: precise and reliable targeting and safety considerations," he explained. "This part of the work is in the hands of our co-authors from LabTau."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.