At the same time that a validated fractional flow reserve CT angiography (FFR-CTA) application seems to be nearing U.S. Food and Drug administration approval, a new contender for gauging the severity of coronary disease at CT is pushing its way into the ring. Furthermore, it promises to be a simpler solution.
Dutch researchers are testing a new onsite fluid dynamics application that promises results similar to the original FFR-CT application but with computational overhead. It promises rapid results that can be processed right in the imaging department rather than sending data off to a remote center and waiting several hours, as the original FFR-CT algorithm requires.
 Dr. Koen Nieman from Erasmus University Medical Center in Rotterdam.
Dr. Koen Nieman from Erasmus University Medical Center in Rotterdam."Both specificity and accuracy were significantly improved with the use of this algorithm," said Dr. Koen Nieman from Erasmus University Medical Center in Rotterdam in a talk at the recent International Symposium on Multidetector-Row CT in San Francisco.
Measuring the functional severity of CT-detected coronary artery stenosis noninvasively is a hot topic these days as researchers look for ways to avoid invasive coronary angiography in patients who turn out not to have needed revascularization after they've already gotten it.
The noncommercial investigative software from Siemens Healthcare is quite new, and, therefore, hasn't been subjected to the level of scrutiny and multicenter investigation that's been accorded to HeartFlow's FFR-CT algorithm for patients with suspected coronary artery disease undergoing coronary CT angiography (CCTA). With study results showing significant improvements in the accuracy of CCTA for determining significant functional stenosis with use of HeartFlow's FFR-CT application -- and by definition improvement in selecting patients for revascularization -- that application is edging toward clinical use. But while accurate, the existing algorithm is computationally intense, requiring radiologists to send their CCTA to HeartFlow's remote lab for processing on supercomputers.
Meanwhile, the investigational algorithm from Siemens aims to make the process easier using a simpler algorithm that can be processed on a laptop computer in a few minutes right in the imaging center.
CCTA just part of the answer
"CTA is excellent for ruling out coronary artery disease, but the positive predictive value still needs some improvement," said Nieman, who is an associate professor and director of cardiovascular imaging research at Erasmus. "In terms of angiography, subjectively we still tend to score in a defensive way," he said, citing studies by Meijboom et al and Tonino et al showing overestimation of stenosis. "But overestimation of angiographic disease is also relevant for CTA. ... Even when you do it invasively along with the gold standard, a majority of patients with 60% to 70% stenosis don't have hemodynamically significant lesions. Even for those in very severe scenarios, there are a substantial proportion of patients who don't have objective ischemia when you perform FFR."
Based on flow dynamics science used to test aircraft and automobiles to model wind resistance, computational flow dynamics has in recent years been applied to the coronary arteries, where it estimates blood flow and pressure within vessels, simulating vasodilation.
"We have been evaluating an algorithm that ... that allows you to do the FFR-CT algorithm yourself," Nieman said. "It's also calculated on a regular workstation and doesn't require a supercomputer. It sort of works on the same principles [as the HeartFlow application]; of course the algorithms are a little bit different and probably a little less complicated."
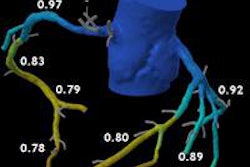
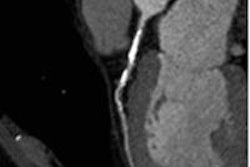
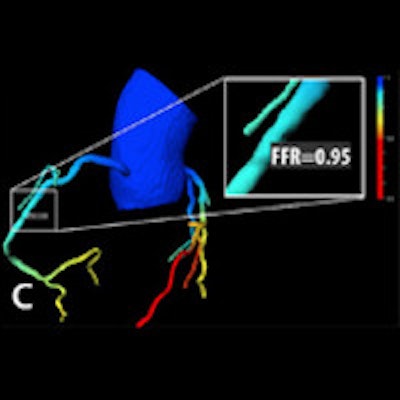
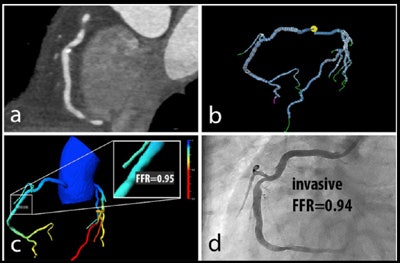 Patient with right coronary artery stenosis visualized on CCTA (a). Investigative FFR-CT application measures stenosis severity at 0.95 (c), confirmed at 0.94 on invasive angiography (d). All images courtesy of Dr. Koen Nieman.
Patient with right coronary artery stenosis visualized on CCTA (a). Investigative FFR-CT application measures stenosis severity at 0.95 (c), confirmed at 0.94 on invasive angiography (d). All images courtesy of Dr. Koen Nieman.The new algorithm processes the data in several steps, beginning with automatic data tracking, then segmentation of the vessels, and finally creating a 3D coronary artery model. The segmentation process takes about 16 minutes on a laptop computer, calculation another 15 minutes per dataset, Nieman said. Similar to the original FFR-CT algorithm, the user must know the left-ventriuclar mass to calculate the blood flow, he said.
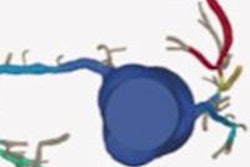
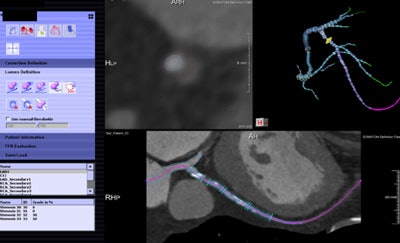 Noncommercial work-in-progress FFR-CT application from Siemens Healthcare estimates blood flow in vessels.
Noncommercial work-in-progress FFR-CT application from Siemens Healthcare estimates blood flow in vessels.In a study originally presented at the 2014 European Congress of Radiology, Nieman and his colleagues at Erasmus examined 106 patients undergoing both coronary CTA and catheter-based FFR. Patients with prior stenting, calcium scores greater than 2000 or poor image quality were excluded.
A total of 189 vessels were segmented using the new algorithm. Patients had diffuse coronary artery disease and a mean coronary artery calcium score of 545, Nieman said. Forty-six percent had stenosis at invasive coronary angiography of greater than 50%, and only 42% had cath-based FFR results less than 0.80.
With the use of the investigational algorithm, CCTA's sensitivity for obstructive disease rose from 81% to 88%, specificity rose from 38% to 65%, and accuracy rose from 56% to 75%, he said, cautioning that sensitivity was on the low side due to use of a functional reference standard. (In its most recent studies, the Heartflow algorithm is logging sensitivities in the mid-80s.)
As well as the new algorithm seems to work, some practical issues remain for clinicians, according to Nieman.
"If the image quality is not good, then we cannot perform these calculations," he noted. And while processing is relatively fast -- 15 minutes for segmentation and 16 minutes on average for calculation of FFR-CT, "most of the time is in preparation," he said.
Hopefully prep will eventually be more completely automated by, for example, eliminating manual drawing of regions for calculation, allowing clinicians to make decisions on the spot with this application, Nieman said.
Just as good as the original FFR-CT algorithm?
In later discussions, Dr. Dominik Fleischmann from Stanford University in the U.S. said that considering the state-of-the-art work with the original FFR-CT algorithm presented by the University of British Columbia's Dr. Jonathon Leipsic from Vancouver just before Nieman's talk, that both algorithms seemed to work well for gauging stenosis severity. But for Leipsic, equivalence was a stretch.
"I've known Dr. Nieman for some time, and I think even he would argue that you really can't simply lump the two [algorithms together] at present in terms of diagnostic performance yet in an academic fashion in the same way," Leipsic said.
The HeartFlow evidence is from a multicenter prospective trial with 750 patients, and while the evidence on the new model "is very thought provoking, because it can be done at the site, and a very compelling initial experience ... I'm not sure we can put the two in the same bin" and say they are equivalent, he said.
Is the four-hour wait for results from a remote center a real drawback? It could be, Leipsic said, but "most of the patients I do FFR-CT on have stable angina with moderate stenosis. In the patient with stable chest pain, "what I want is accurate FFR, and if I have to wait four hours, if I have to wait a day, it's not an issue," he said. In the setting of acute chest pain, however, "I agree that four hours is frustrating, though probably in a troponin-negative patient it's not a huge issue," Leipsic said.
"If I could get [FFR results] in my hospital, if I could get it the same day, of course it would be great," Leipsic continued. "But I have concerns about how accurate that [new algorithm] model could be."