New tracers and imaging techniques for the assessment of heterogeneity are emerging fast, especially in the context of examining metastasis and predicting metastatic potential, but there is a significant need for large patient trials and applications to fully determine their specific validity in the personalized patient treatment paradigm, according to researchers at a top U.K. facility.
"Tumor heterogeneity has, in recent times, come to play a vital role in how we understand and treat cancers; however, the clinical translation of this has lagged behind advances in research. Although significant advancements in oncological management have been made, personalized care remains an elusive goal," noted Ruhe Chowdhury and colleagues from the Randall Division of Cell and Molecular Biophysics at King's College London and the Department of Medical Oncology at Guy's and St Thomas' National Health Service (NHS) Foundation Trust in London.
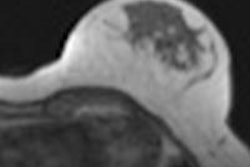
![Simultaneous F-18 FDG-PET/MRI-acquired image of a patient with a sigmoid tumor. Fused axial T2 and PET (a), PET alone (b), MRI apparent diffusion coefficient map (c) and representative subtracted image from a dynamic contrast-enhanced MRI series (d) show increased metabolism, cellularity, and vascularity. Produced from an imaging unit at the Institute of Nuclear Medicine, University College London. Figures republished with permission of British Institute of Radiology, from [Chowdhury R, Ganeshan B, Irshad S, Lawler K, Eisenbl¨atter M, Milewicz H, et al. The use of molecular imaging combined with genomic techniques to understand the heterogeneity in cancer metastasis. Br J Radiol, 2014;87:20140065.]](https://img.auntminnieeurope.com/files/base/smg/all/image/2014/05/ame.2014_05_29_09_17_16_675_2014_05_29_molecular_BJR-29may-fig4-high-res.png?auto=format%2Ccompress&fit=max&q=70&w=400) Simultaneous F-18 FDG-PET/MRI-acquired image of a patient with a sigmoid tumor. Fused axial T2 and PET (a), PET alone (b), MRI apparent diffusion coefficient map (c) and representative subtracted image from a dynamic contrast-enhanced MRI series (d) show increased metabolism, cellularity, and vascularity. Produced from an imaging unit at the Institute of Nuclear Medicine, University College London. Figures republished with permission of British Institute of Radiology, from [Chowdhury R, Ganeshan B, Irshad S, Lawler K, Eisenbl¨atter M, Milewicz H, et al. The use of molecular imaging combined with genomic techniques to understand the heterogeneity in cancer metastasis. Br J Radiol, 2014;87:20140065.]
Simultaneous F-18 FDG-PET/MRI-acquired image of a patient with a sigmoid tumor. Fused axial T2 and PET (a), PET alone (b), MRI apparent diffusion coefficient map (c) and representative subtracted image from a dynamic contrast-enhanced MRI series (d) show increased metabolism, cellularity, and vascularity. Produced from an imaging unit at the Institute of Nuclear Medicine, University College London. Figures republished with permission of British Institute of Radiology, from [Chowdhury R, Ganeshan B, Irshad S, Lawler K, Eisenbl¨atter M, Milewicz H, et al. The use of molecular imaging combined with genomic techniques to understand the heterogeneity in cancer metastasis. Br J Radiol, 2014;87:20140065.]Inter- and intratumor heterogeneity, particularly in the clinical setting, has been difficult to quantify and therefore to treat, and the ability to examine not only the whole tumor, but also the molecular variations of metastatic disease in a patient remains challenging. However, advances in imaging techniques and novel applications, along with increased understanding of tumor heterogeneity, have opened up a plethora of noninvasive biomarker potential to examine tumors, their heterogeneity and the clinical translation, they explained in the June issue of the British Journal of Radiology (Br J Radiol, Vol. 87:1038, pp. 20140065).
"Although the use of systemic nontargeted and targeted adjuvant therapies has helped to prevent the spread of tumor cells from the primary site and is now a standard practice for many tumor types, the emergence of resistant disease continues to be a significant cause of patient mortality. These features provide an insight into the dynamic nature of the signaling network within the tumor cells, and human cancers are now being increasingly recognized as heterogeneous, characterized by distinct pathological, genomic, clinical, and therapeutic features," wrote the authors.
CT is widely used for tumor assessment, but provides very little in the way of distinct tumor activity information; adding PET to CT can add such information, and F-18 FDG is the most commonly used PET radiotracer, although there are many other radiotracers that examine different aspects of tumor biology, they stated. Although primarily reporting on tumor cell activity, F-18 FDG-PET has been shown to also inform on tumor heterogeneity.
The addition of MR to PET can add heterogeneity information about the tumor phenotype that is gathered from radionuclide-based studies. Dynamic contrast-enhanced (DCE) imaging differs from traditional MRI through the ability to acquire multiple images, before, during, and after contrast injection, and in PET/MRI, the technique allows dynamic imaging of tumors to take place, with detailed imaging of tumor vascularity through the concomitant evaluation of αvβ3 expression and high glucose metabolism within tumors that can show perfusion heterogeneity, they explained. This form of imaging has also played a role in treatment assessment with vascular endothelial growth factor inhibitor use.
"The combination of microstructural and vascular information afforded by MRI with specific metabolic PET tracers can now be achieved in a clinic with whole-body PET/MR scanners," Chowdhury et al noted. "Multiparametric imaging has well-recognized utility for microstructural and vascular tissue characterization and is rapidly establishing an expanding niche in the localization and management of prostate cancer. Yet, in general, it remains more difficult to assess metabolic activity with MRI than with PET."
','dvPres', 'clsTopBtn', 'true' );" >
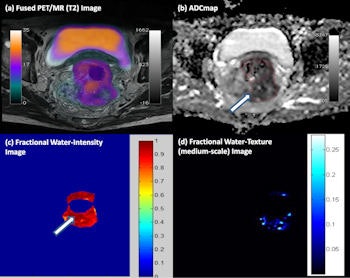
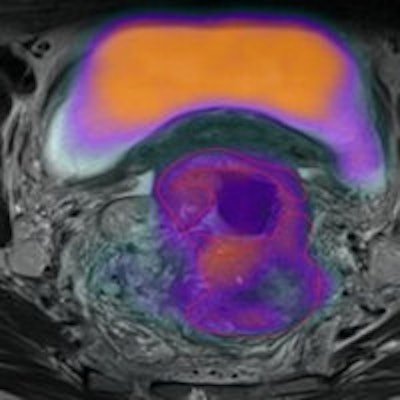
Click image to enlarge.
Multiparametric PET/MRI of a rectal cancer. (a) High F-18 FDG uptake on fused PET/T2 MRI, with (b) a correspondingly patchy reduced apparent diffusion coefficient in keeping with pockets of high cellularity within the tumor and (c) a fractional water image derived from source fat and water Dixon images of the same tumor confirms that areas of increased cellularity correlate with relatively increased water content (white arrows). (d) Application of a medium coarse textural filter highlights 3- to 4-mm bright objects on the fractional water image (medium texture map).
Multiparametric PET/MR performed by the authors has demonstrated the ability to assess glycolysis, cellularity, and water content and intralesional heterogeneity (via texture analysis) within a single examination (see figure). They found that tumors with more heterogeneous water distribution (i.e. higher standard deviation and proportion of positive pixels) were more cellular (i.e. lower mean apparent diffusion coefficient) and glycolytic (i.e. higher SUVmean).
Foci of high cellularity also correspond to areas of increased glycolysis. Textural filters applied to the fractional water images revealed features of around 3- to 4-mm bright objects, which may be associated with pockets of water content and tended to be higher within tumors having adverse biology (restricted diffusion and increased glucose uptake).
Multiparametric PET/MRI datasets evaluating tissue microstructure, metabolism, and heterogeneity are likely to contain prognostic information/relate to metastatic potential, but both hypotheses require further validation, they emphasized.
Simultaneous PET/MRI offers the opportunity in the clinic to combine tissue characterization multiparametric MRI with specific molecular PET imaging, with the potential to assess dynamic biological relationships through multimodal imaging of, for example, tumor cellularity/cell turnover [diffusion-weighted imaging (DWI) or FLT-PET], hypoxia (blood oxygen level-dependent MRI or F-18 fluoromisonidazole PET ligand), vascularity (DCE/MRI or α-V-β-3 PET ligand) and glycolysis (18F-FDG-PET ligand or glucose chemical exchange saturation transfer MRI).
Looking to the future, the need for robust validation is crucial to offering patients lasting results.
"The role of clinical trials should not be purely to review efficacy of treatment but also to aid the development of new research. To this end, the acquisition of patient samples at each step of the treatment paradigm plays a vital role in developing the translational application of research," the authors concluded. "The integration of functional imaging, patient sampling, and drug development together with wider research is likely to play a key role in fully understanding the nature of heterogeneity and ultimately how to control its effects to clinical advantage."
Study disclosures
Two of the authors (B. Ganeshan and K.A. Miles) are shareholders in TexRAD, a company developing and marketing the commercial (research) texture analysis software. This work was supported by KCL Breakthrough Breast Cancer Research Unit/Sarah Green Fellowship funding and an endowment fund from Dimbleby Cancer Care to King's College London (TN). Support also came from the KCL-UCL Comprehensive Cancer Imaging Centre, an FP7-HEALTH-2010 European Union grant titled "IMAGINT" (grant no. 259881), and the U.K. Department of Health's NIHR Biomedical Research Centre. The first three authors, Ruhe Chowdhury, Balaji Ganeshan, PhD, and Sheeba Irshad, contributed equally to this article.