MRI scans have confirmed that the drug fingolimod reduced inflammatory lesion activity and brain volume loss in patients with multiple sclerosis (MS). Results from the two-year, placebo-controlled trial were published online on 2 July in Archives of Neurology.
The beneficial anti-inflammatory effects of fingolimod therapy were seen at MRI as soon as six months after treatment began and were sustained over the two years. Approximately 50% of patients receiving fingolimod therapy experienced no new inflammatory lesions throughout the study, compared with 21% of patients who received the placebo.
Fingolimod is marketed as Gilenya by pharmaceutical firm Novartis. It was approved by the U.S. Food and Drug Administration (FDA) in September 2010 as a once-a-day dose of 0.5 mg to 1.5 mg to treat relapsing multiple sclerosis.
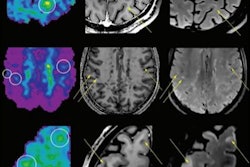
MRI can help detail the inflammatory progression of MS, as seen in gadolinium-enhancing lesions on T1-weighted images or new and enlarging T2 lesions. The extent of hyperintense areas on T2-weighted MR images indicates overall disease burden, the authors noted.
FREEDOMS trial
The study was led by Dr. Ernst-Wilhelm Radue from the Medical Image Analysis Center at University Hospital Basel. The research included 1,272 patients who were part of the Fingolimod Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) trial. The randomized, phase III trial took place at 138 centers in 22 countries from January 2006 to July 2009 (Arch Neurol, 2 July 2012).
Patients received either fingolimod capsules of 0.5 mg or 1.25 mg once per day or placebo. The group ranged in age from 18 to 55 years and had a confirmed diagnosis of multiple sclerosis. Patients were included in the study if they had one or more documented relapses in the previous year or two or more relapses in the previous two years.
Exclusion criteria included being treated with steroids within the previous 30 days, drug- or disease-induced immune suppression, an inability to undergo MRI due to claustrophobia, or an allergy to gadolinium-based contrast.
The researchers performed MRI scans at baseline, six, 12, and 24 months. At each point, T1- and T2-weighted images were obtained before and after contrast administration. The researchers identified lesions and calculated their volume, as well as the percentage of brain volume change among the patients from their baseline scans.
Lesion and volume reduction
Of the 1,272 patients, 1,033 (81%) completed the 24-month study, with 945 (74%) receiving fingolimod during the duration of the trial.
Fingolimod doses of 0.5 mg or 1.25 mg both reduced the number of new and newly enlarged T2 lesions over 24 months compared with placebo, according to the authors. Reductions in T2 lesions were deemed "significant" at six months and remained throughout the 24-month study period.
Those who received either dose of fingolimod also had fewer gadolinium-enhanced lesions and reduced gadolinium-enhanced lesion volume at each MRI scan over the two years, compared with the placebo-treated patients.
There was also less MS progression in patients treated with fingolimod, as shown by T2 lesion volume over the study period. T2 lesion volume had decreased slightly from baseline at 12 and 24 months for both fingolimod dosage groups, while volume increased among the placebo patients.
In terms of brain volume change, both fingolimod dose levels reduced the mean percentage of brain volume change during the 24-month period, compared with the placebo group.
"This reduction in [percentage of brain volume change] was significant by month 6 and was sustained during months 0 to 12 and 12 to 24," the authors noted. "The relative reduction in brain volume loss for fingolimod, relative to placebo, ranged from 23% to 45% at the various intervals."
The results confirm the efficacy of fingolimod in MS patients, Radue and colleagues concluded.
"These analyses from the two-year FREEDOMS study confirm that the efficacy of fingolimod therapy is robust across all MRI end points," they wrote. "The anti-inflammatory effects of fingolimod therapy, as depicted by gadolinium-enhancing lesions and new/newly enlarged T2 lesions, were evident as early as six months after treatment initiation and were sustained over two years."
|
Study disclosures The study was funded by Novartis. |