Integrating MR elastography (MRE) in a routine MRI scanner and combining it with dynamic contrast-enhanced MRI (DCE-MRI) could predict complete pathological response at the end of neoadjuvant chemotherapy in breast cancer patients, according to a presentation at the International Society for Magnetic Resonance in Medicine (ISMRM) annual meeting.
Researchers led by Aish Sinha, MBBS, from King’s College London in England demonstrated how combining DCE-MRI with MRE achieved high sensitivity and specificity in predicting complete pathological response.
“Quantifying biomechanics throughout chemotherapy via MRE in conjunction with DCE-MRI in breast cancer patients undergoing primary chemotherapy yields more accurate results in predicting complete pathological response than current international imaging gold standard methods,” Sinha said.
DCE-MRI is the gold standard for monitoring tumor response in breast cancer patients who undergo neoadjuvant chemotherapy, Sinha highlighted. However, he added that there is a need to identify alternative, non-invasive imaging biomarkers to find tissue alterations related to tumor response mechanisms.
The researchers studied whether biomechanics quantified over time with MRE can identify complete pathological response in patients undergoing chemotherapy, as well as predict response at an earlier time point throughout chemotherapy.
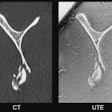
They integrated MRE into a biopsy coil (Siemens Healthineers) for integration of data gathering within standard breast MRI. The team acquired data using a 1.5-tesla MRI system (Aera, Siemens throughout a seven-minute scan using a 3D MRE gradient echo sequence. The team noted that this allowed for non-invasive quantification of tissue biomechanics over 16 consecutive slices at 36Hz mechanical vibration with an isotropic spatial resolution of 3 mm.
The study included 41 women with 205 individual scans. Scans were performed before (1.1) and during (2.1) chemotherapy.
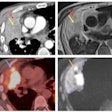
Women with a complete pathological response showed a drop in elasticity, while patients with a partial response had a constant or increased elasticity. Additionally, pathological response depended on tumor type (Chi2 test, p = 0.004). This included a complete pathological response more likely to be found in triple-negative breast cancer and HER2+/ERPR- breast cancer.
The researchers found that relative elasticity was the most accurate individual predictor of histopathological response to treatment. However, it was not as sensitive as DCE-MRI.
The team also used a decision tree to find out whether a combined biomarker approach was more accurate than MRE and DCE-MRI alone when the elasticity ratio was borderline (0.95-1.05). It reported that this approach led to increased sensitivity from 77.8% to 94.4% while maintaining the high specificity of MRE alone (95.7% compared with 91.3%).
| Comparison between MRI approaches in predicting tumor response | |
|---|---|
| MRI method | Receiver operator curve value |
| Combined MRE/DCE-MRI | 0.95 |
| MRE | 0.84 |
| MRI | 0.69 |
DCE-MRI and MRE combined achieved a sensitivity of 94.4% and a specificity of 95.7% in predicting complete pathological response.
Finally, the researchers reported that at the early 1.1 scan time point, response/resistance correlated with a corresponding rise/drop in phase angle.
“It is important to remember that response/resistance is determined via histopathology at end-neoadjuvant chemotherapy,” Sinha said. “As such, it is intriguing that the relative change in phase angle carries such early predictive information [p < 0.0001].”
Sinha highlighted that the combined approach is “a promising new concept” that could serve as a noninvasive diagnostic tool in determining whether surgery should be de-escalated.