Low-field MRI can now detect many pathologies in the lungs that previous generations of MRI scanners failed to do, but there is still significant room for improvement once radiologists become more proficient at interpreting pulmonary 0.55-tesla (0.55T) MRI scans, researchers from California have reported at the International Society for Magnetic Resonance in Medicine (ISMRM) 2024 meeting.
"MR technology has matured to a tipping point in which the field of lung MR that used to be a largely research-only endeavor is now becoming more relevant in our daily clinical practice. However, despite the improvement, technology is still several milestones away from being fully mature and carries inherent technical limitations of MRI as well," Felicia Tang, from the School of Medicine, University of California, San Francisco (UCSF), told AuntMinneEurope.com ahead of her ISMRM presentation on 7 May.
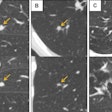
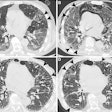
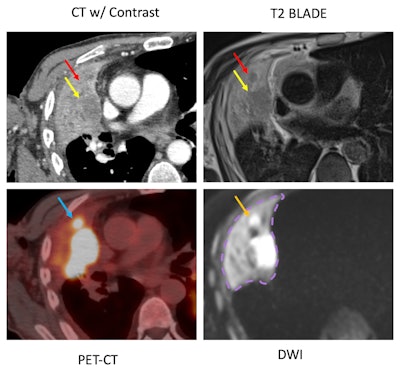 Example case shows advantages of DWI sequence in detecting a mass hidden inside atelectasis. CT chest, PET-CT, T2 BLADE, and DWI are compared for a 72-year-old male patient with a satellite nodule (red arrow) obscured by atelectasis adjacent to the dominant right upper lobe mass (yellow arrow) in CT. However, DWI image demonstrates restricted diffusion (orange yellow) corresponding to focal FDG uptake (blue arrow) within the satellite nodule.
Example case shows advantages of DWI sequence in detecting a mass hidden inside atelectasis. CT chest, PET-CT, T2 BLADE, and DWI are compared for a 72-year-old male patient with a satellite nodule (red arrow) obscured by atelectasis adjacent to the dominant right upper lobe mass (yellow arrow) in CT. However, DWI image demonstrates restricted diffusion (orange yellow) corresponding to focal FDG uptake (blue arrow) within the satellite nodule.
Radiologists who know the pros and cons of low-field MRI will be able to make more confident recommendations to clinicians and offer this emerging modality to the appropriate patients. "This is an exciting time for 0.55T lung MRI, with many more clinical indications that will emerge in the next several years from multiple lung MR investigators conducting studies throughout the world," she said, noting that functional ventilation/perfusion imaging looks set to complement the anatomic lung capabilities of 0.55T MRI.
Larger pathologies like consolidations are easily detectable by MRI, but smaller pathologies like small nodules under 7 mm or air-filled pathologies like emphysema are still difficult to detect, according to Tang, who said the main surprise in the UCSF study was that quite a few cases were not detected by radiologists prospectively but were retrospectively visible when the data were analyzed. "Pulmonary MRI has not been the imaging modality of choice for lung imaging and there is a need for training to make sure our eyes are well trained for the new modality."
The group prospectively enrolled 28 patients with heterogeneous pulmonary pathologies encompassing benign nodules, malignancies, infections, bronchiectasis, and interstitial lung diseases, who concurrently underwent same-day CT or PET/CT. A 0.55T scanner (Magnetom Free.Max, Siemens Healthineers) was used with respiratory-navigated T2-weighted BLADE (coronal and axial), T1-weighted ultrashort echo time (UTE) with spiral readout, and diffusion-weighted imaging (DWI) sequences (both UTE and DWI using research applications).
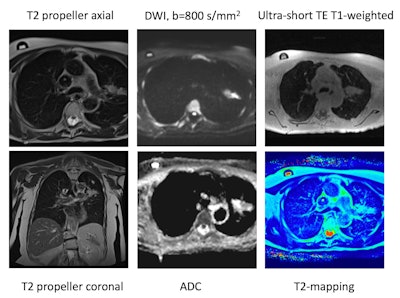 0.55T lung MRI protocol. Example protocol includes respiratory-navigated T2 BLADE breath-triggered (coronal and axial), 3D respiratory-navigated T1 ultrashort echo time (UTE), diffusion-weighted imaging (DWI) sequences, and T2-mapping, with the potential of adding susceptibility weighted imaging and other sequences in the future. All figures courtesy of Felicia Tang et al and presented at ISMRM 2024.
0.55T lung MRI protocol. Example protocol includes respiratory-navigated T2 BLADE breath-triggered (coronal and axial), 3D respiratory-navigated T1 ultrashort echo time (UTE), diffusion-weighted imaging (DWI) sequences, and T2-mapping, with the potential of adding susceptibility weighted imaging and other sequences in the future. All figures courtesy of Felicia Tang et al and presented at ISMRM 2024.
The maximum diameter of the ascending aorta, descending aorta, left and right branch pulmonary arteries, main pulmonary artery, mid-thoracic trachea, and sternal thickness were measured with CT and T2 BLADE axial sequence. Representative lymph nodes, nodules of varying sizes, and consolidations described on the CT report were measured with CT and T2 BLADE Axial, or UTE if not visible on T2 BLADE Axial. Measurements were compared using coefficient of determination (R2) with significant correlation defined as p < 0.05. Mean of absolute difference (MAD) was also calculated.
As part of a reader performance study, six radiologists independently read all corresponding CT images and then graded the overall image quality of MRI images on a 3-point Likert-type scale (1 = low, 2 = moderate, 3 = good). Intraclass correlation coefficient was calculated for the standardized overall image quality gradings. After a four-week wash out period, two readers identified the presence of common lung pathologies using only 0.55T MRI. Sensitivity was calculated as the percentage of major diagnoses reported on CT that were identified and visible to the reader on MRI by each pathology category.
The team observed an overall correlation of R2 = 0.89 for the ascending aorta, 0.79 for the descending aorta, 0.73 for the main pulmonary artery, 0.74 for the right pulmonary artery, 0.27 for the left pulmonary artery, 0.93 for the mid-thoracic trachea diameter, and 0.81 for the sternal thickness. The MAD was the lowest for mid-sternal thickness at 0.63 mm and highest for right pulmonary artery at 2.4 mm. For 24 pathologies measured (solid nodule = 15, subsolid nodule = 1, cavitary consolidation = 1, mass = 2, consolidation = 1, mediastinal lymph node = 4), overall R2 = 0.95, and MAD = 1.5 mm.
The results of the reader performance study demonstrated a mean of 2.2 and a standard deviation of 0.5 for overall image quality grading. Intraclass correlation was 0.44 after standardizing the image quality gradings. Sensitivity of pathology identification using 0.55T MRI was highest for consolidation (Reader 1) and reticulation (Reader 2). Both readers were unable to identify emphysema and pulmonary artery dilation, and had trouble identifying pulmonary nodules smaller than 8 mm. Thus, CT is better for detection of small nodules < 8 mm, but 0.55T MRI also has its strengths and potential applications, particularly for tumor recurrence detection.
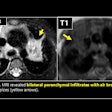
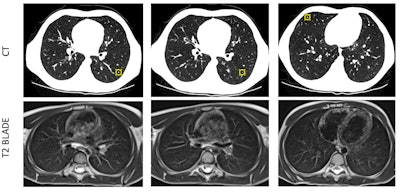 CT has an advantage in detection of small pulmonary nodules compared to 0.55T MRI. A 7-year-old boy's three small pulmonary nodules (3 mm, 2 mm, and 3 mm) in bilateral lungs were detected on CT but not on 0.55T MRI. CT is still superior for detection of small nodules -- a limitation of 0.55T MRI.
CT has an advantage in detection of small pulmonary nodules compared to 0.55T MRI. A 7-year-old boy's three small pulmonary nodules (3 mm, 2 mm, and 3 mm) in bilateral lungs were detected on CT but not on 0.55T MRI. CT is still superior for detection of small nodules -- a limitation of 0.55T MRI.
Although MRI has had low adoption in lung imaging due to susceptibility artifacts, limiting its utility in pulmonary parenchymal imaging, low-field MRI (0.55T) those limitations can be overcome, but its thoracic diagnostic capabilities remain indeterminate, the researchers noted. Although MRI for lung imaging has had challenges due to image degradation, the 0.55T MR system has the advantage of reduced susceptibility artifact at the air-tissue interface while also offering modern image acquisition and respiratory navigation technologies.
"One of the primary purposes of our study was to open up the most appropriate and valuable follow-up research studies," noted Tang. "Technically, even though the average image quality has improved substantially from prior generations of MRI, there remains significant variation in image quality that needs to be addressed to maximize the clinical usability."
Since the poster was submitted, the authors have completed the reader performance study and have focused their analysis of the results based on categorizing the pathologies into different visibility levels on 0.55T MRI. A pathology is categorized as excellent visibility if 4-6 readers correctly identified it on MRI; intermediate visibility if 2-3 readers correctly identified on MRI; and poor visibility if 0-1 readers correctly identified on MRI. "We are also working on understanding why certain pathologies had intermediate visibility on MRI," Tang added.
The co-authors of the ISMRM 2024 poster are Timothy Chen, Sayedomid Ebrahimzadeh, Brandon K.K. Fields, Jonathan Liu, Yoo Jin Lee, Adam Yen, Kiara Bowers, Peder Larson, Yang Yang, and Jae Ho Sohn, all from UCSF, and Brandon Schonour and Pan Su from Siemens Healthineers.