Radiopharmaceutical firm Piramal Imaging announced that its NeuraCeq radiotracer has received marketing authorization from the European Commission (EC).
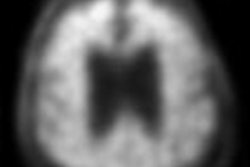
NeuraCeq is a radiopharmaceutical indicated for PET imaging of beta-amyloid neuritic plaque density in the brains of adult patients with cognitive impairment who are being evaluated for Alzheimer's disease (AD) and other causes of cognitive impairment.
NeuraCeq will be available in select European countries in the second and third quarters of 2014.
Also, Piramal has partnered with IBA Molecular for manufacturing and distribution of NeuraCeq upon European Union approval. IBA Molecular owns and operates a network of 49 PET isotope facilities worldwide.