Eli Lilly subsidiary Avid Radiopharmaceuticals has received marketing authorization from the European Commission for its Amyvid (florbetapir F-18) PET radiotracer.
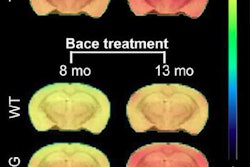
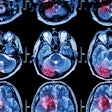
Amyvid received clearance as a diagnostic radiopharmaceutical indicated for PET imaging of beta-amyloid neuritic plaque density in the brains of adult patients with cognitive impairment who are being evaluated for Alzheimer's disease and other causes of cognitive impairment, according to the company.
Amyvid will be available in select areas within the European Union beginning in the second quarter. It's supplied in 10 mL, 30 mL, or 50 mL multidose vials containing 500-1,900 MBq/mL florbetapir F-18.