A preclinical study from Belgium has shown promising results with the PET tracer florbetapir for targeting amyloid plaque and tau tangles in the brain and potentially evaluating the efficacy of certain Alzheimer's treatments. Findings were published in the December issue of the Journal of Nuclear Medicine.
Approximately 10% of people older than 65 have Alzheimer's dementia and more than 5 million in the U.S. are living with the disease, according to the Alzheimer's Association. The number of people with Alzheimer's is projected to increase to 16 million by 2050.
Previous studies have shown that PET scans of the brain can identify the accumulation of beta-amyloid plaque and tau tangles that are associated with the onset of cognitive impairment, dementia, and Alzheimer's disease.
With that foundation, researchers from the Molecular Imaging Center Antwerp, led by Steven Deleye, PhD, are conducting a large-scale longitudinal micro-PET study using three tracers in mice models. Their goal is to evaluate the effects of treatment with an inhibitor of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1), which cuts off protein fragments that can lead to beta-amyloid development (JNM, December 2017, Vol. 58:12, pp. 1977-1983).
Using the PET tracers florbetapir (Amyvid, Eli Lilly), FDG, and PBR111, the researchers compared a group of normal control mice with mice genetically altered to have Alzheimer's. The micro-PET imaging protocol consisted of the following:
- A 45-minute uptake period and 20-minute scan with FDG
- A 40-minute uptake period and 20-minute scan with PBR111
- A 30-minute uptake period and 20-minute scan with florbetapir
The mice received the BACE inhibitor at seven weeks, at which time the researchers performed baseline micro-PET scans to measure brain metabolism, neuroinflammation, and beta-amyloid pathology for each of the three tracers.
Follow-up scans were performed at four, seven, and 12 months, and florbetapir uptake was measured at 8 and 13 months of age. Microscopic studies were conducted after the last exams.
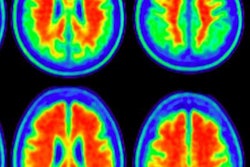
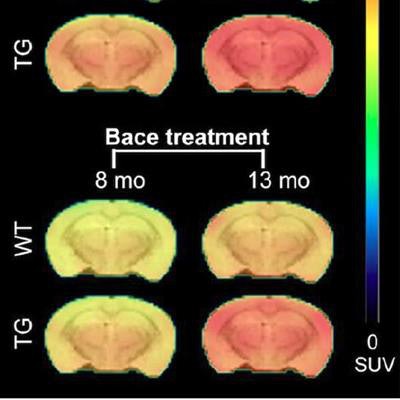
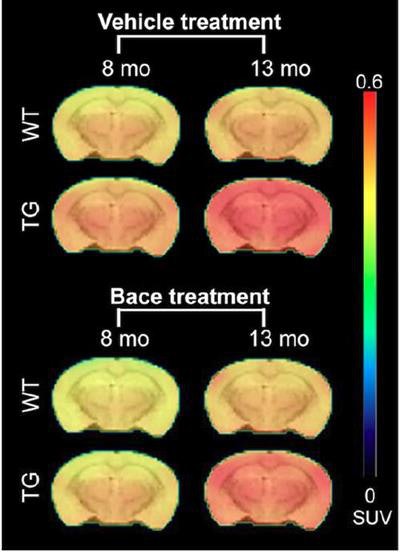 PET shows the average florbetapir uptake per mouse group at 8 and 13 months of age. A significant interaction of genotype treatment was observed in the cortex (p = 0.0248), hippocampus (p = 0.0071), and thalamus (p = 0.0084), indicating reduced florbetapir uptake in BACE1-inhibited mice. WT = wild type (controls); TG = transgenic (genetically modified). Image courtesy of the Molecular Imaging Center Antwerp and JNM.
PET shows the average florbetapir uptake per mouse group at 8 and 13 months of age. A significant interaction of genotype treatment was observed in the cortex (p = 0.0248), hippocampus (p = 0.0071), and thalamus (p = 0.0084), indicating reduced florbetapir uptake in BACE1-inhibited mice. WT = wild type (controls); TG = transgenic (genetically modified). Image courtesy of the Molecular Imaging Center Antwerp and JNM.All three tracers detected pathological differences between the genetically modified mice and the controls; however, only florbetapir illustrated the effects of inhibitor treatment by identifying reduced beta-amyloid pathology in the genetically modified mice, as confirmed by the microscopic studies.
"This study clearly showed that accurate quantification of [beta-amyloid] tracers is critically important and that the nonspecific uptake in the brain of subjects might be underestimated for some existing Alzheimer's tracers that have fast metabolization profiles," the authors wrote.