Altered glucose metabolism is associated with a range of pathological conditions. In particular, solid tumors exhibit increased glucose consumption compared with normal healthy tissue -- a feature that can be exploited for tumor detection, monitoring disease progression, and evaluating response to therapy, U.K. researchers have reported.
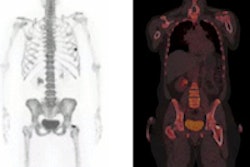
Glucose uptake is currently assessed using F-18 FDG PET. However, this necessitates introduction of radiolabeled glucose analogues to the patient, thereby limiting the ability to perform repeated, longitudinal measurements. It also precludes use in certain patient populations such as young children or pregnant women.
Now, researchers at University College London (UCL) have developed an alternative MRI-based method for imaging glucose uptake and metabolism in vivo. The new approach -- called glucose chemical exchange saturation transfer (glucoCEST) -- does not require the use of ionizing radiation and instead images unlabeled glucose at physiologically reasonable quantities (Nature Medicine, 7 July 2013, online first).
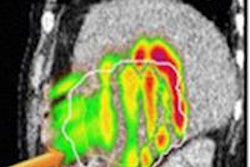
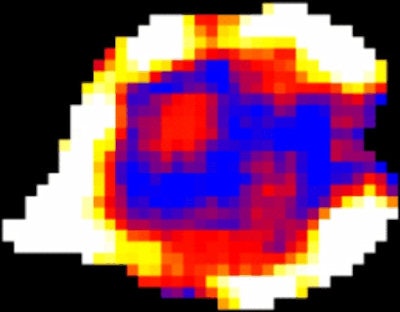 Glucose uptake varies within tumors, as demonstrated using a new technique developed by scientists at UCL. "Hot" regions at the edge of the tumor show increased uptake compared with "cold" central regions, which could be used in the future to determine the best therapies to give to individual patients. Image courtesy of UCL.
Glucose uptake varies within tumors, as demonstrated using a new technique developed by scientists at UCL. "Hot" regions at the edge of the tumor show increased uptake compared with "cold" central regions, which could be used in the future to determine the best therapies to give to individual patients. Image courtesy of UCL."GlucoCEST uses radiowaves to magnetically label glucose in the body," explained lead researcher Simon Walker-Samuel, PhD, from the UCL Centre for Advanced Biomedical Imaging (CABI). "This can then be detected in tumors using conventional MRI techniques. The method uses an injection of normal sugar and could offer a cheap, safe alternative to existing methods for detecting tumors, which require the injection of radioactive material."
GlucoCEST works by measuring glucose uptake through the chemical exchange of protons between glucose hydroxyl groups and water. By selectively saturating the magnetization of protons in the hydroxyl groups, using radiofrequency pulses, this exchange of protons causes a reduction in the MR signal from water.
The change in the MRI water signal is visualized using the asymmetric magnetization transfer ratio curve. For this study, Walker-Samuel and colleagues defined a new parameter: glucoCEST enhancement (GCE), the change in area under this curve from baseline to after glucose injection.
To assess its in vivo performance, the researchers performed glucoCEST imaging in mice transplanted with two types of human colorectal cancer cells: LS174T and SW1222 (which have markedly different phenotypes). They acquired images from a slice through each tumor, at baseline and 60 minutes after intraperitoneal injection of glucose.
The images revealed a significantly greater GCE in SW1222 tumors compared with muscle. A significant difference in the median GCE between the two tumor types was also seen, indicating that glucoCEST can discriminate between differing tumor phenotypes.
One day after these tests, the researchers administered F-18 FDG to the mice and then performed autoradiography on tumor slices corresponding to the MR imaging slices. In agreement with the glucoCEST experiments, the median activity at 60 minutes after F-18 FDG administration was significantly higher in SW1222 than LS174T tumors (indicating lower glucose uptake in LS174T than SW1222).
The researchers saw a statistically significant correlation between the median tumor GCE and FDG uptake values, providing a clear validation of the glucoCEST technique. F-18 FDG images acquired at 60 minutes after injection showed similar spatial patterns of uptake to the GCE images.
Clinical application
Scaling the dose used in this study to a 70 kg human would correspond to 14 g of glucose. The authors note that oral administration would be easier to implement in the clinic than intravenous injection, possibly by the patient drinking a sugar solution whilst in the MR scanner.
"We can detect cancer using the same sugar content found in half a standard-sized chocolate bar," said Mark Lythgoe, PhD, director of CABI and a senior author on the study. "Our research reveals a useful and cost-effective method for imaging cancers using MRI -- a standard imaging technology available in many large hospitals. In the future, patients could potentially be scanned in local hospitals, rather than being referred to specialist medical centers."
The team is now evaluating glucoCEST in a number of patient groups to evaluate its sensitivity on clinical scanners. "To mirror our experiments in mice, we are administering glucose via a sugary drink in the MRI scanner," Walker-Samuel said. "If sufficiently sensitive, glucoCEST could complement FDG-PET, or provide a cost-effective and safer alternative, potentially with a greater spatial resolution. It could also be used to assess response to therapy or to characterize individual tumor pathophysiology for treatment stratification."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.