To be effective in clinical practice, functional and molecular imaging (FMI) biomarkers in oncology require a stable platform, broad availability, readily specifiable acquisition parameters, standardized interpretation, and a defined reproducibility that has been documented in many cancers and normal ranges.
That's the view of a multinational group of researchers led by Dr. Roberto García-Figueiras, from the department of radiology, University Hospital of Santiago de Compostela, Spain. Their e-poster was given a cum laude award -- the second highest category of prizes -- during RSNA 2012 in Chicago.
The main future challenges include standardization, particularly in terms of acquisition, analysis, and reports, the researchers believe. Reproducibility at an individual and cohort level is also important, as is quality control, universalization of available noninvasive technology, and how to represent tumor heterogeneity (histograms, fractal analysis, and functional parametric maps).
The authors define a biomarker (BM) as a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. Molecular markers are obtained by removing a sample from a patient, and they can detect genetic, genomic, and protein analytes from biofluids or tissue samples. Biomarker technologies, on the other hand, detect and analyze electromagnetic or acoustic biosignals emitted by tissues.
"Imaging BMs can interrogate a large extent of the pathological tissue in vivo, in a relatively noninvasive exam, and check different tumor levels (molecular, functional, etc.) and tumor hallmarks. They can promptly detect small and early tumor response and can be followed up frequently," they pointed out. "However, the quantity and validity of the imaging measurement as a BM often depends crucially on the use of a diagnostic imaging device."
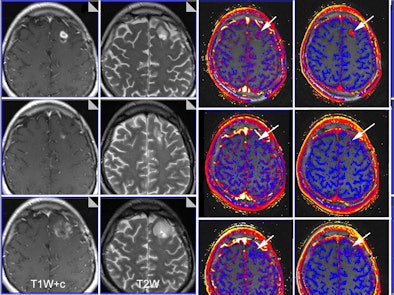 |
| Functional imaging biomarkers can provide important clinical information. In this case, multiparametric MRI was used to evaluate brain glioma response to bevacizumab therapy. Columns: T1-weighted images with contrast; T2-weighted images; transfer constant and leakage space. Rows (from the top): baseline, 10 days of therapy, and six weeks of therapy. Note that morphology imaging shows an initial response by the 10th day, but the tumor then progresses and is much larger at six weeks. However, functional images show that the drug is still working (reductions of transfer constant and leakage space - arrows). Images courtesy of Dr. Anwar Padhani, Paul Strickland Scanner Centre, Mount Vernon Cancer Centre, London, who was a co-author of the RSNA 2012 e-poster. The images have been published in part in the following article: Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010; Vol. 256:2, pp. 348-364. |
BMs are necessary because morphologic imaging does not always allow us to predict who will respond, or who is responding, to therapy. Also, key biological tumor hallmarks are not evaluated using conventional imaging techniques, and tumor size is a late and inaccurate measurement for tumor response and nonmeasurable disease (i.e., peritoneal and bone) may represent the most important portion of tumor burden. Size change does not necessarily reflect therapeutic efficiency, and new therapies are often cytostatic and may not change tumor size, they added.
"Why do we need FMI biomarkers?" García-Figueiras and his colleagues asked. "First, to increase objectivity of assessments and provide a quantitative approach. Second, morphologic appraisals have significant limitations for patient decision-making in the modern era. Third, to evaluate tumor hallmarks (angiogenesis, metabolism, or proliferation). Fourth, anatomic evaluations are insensitive to changes that inform on success of new therapy approaches."
Doctors must be familiar with the following known biological basis of imaging observations, recognized method(s) for quantification, and what the BM means in pre- and post-therapy settings. Imaging BMs can represent tumor biology, but imaging may translate the tumor over expression of proteins (radioproteomics), they explained. BMs can help to evaluate tumor biology in a quantitative way; the radiomics hypothesis involves inferring proteogenomic and phenotypic information from radiological images in a quantitative way.
"In the evaluation of the change of a parameter post-therapy, time needs to be kept in mind. The wrong timing might easily lead to wrong conclusions regarding the efficacy of a certain drug or therapy," wrote the authors.
The direction and magnitude of changes in a BM depend on the mechanism of action of the therapy and corresponding reaction of the tumor/normal tissue to it. Therefore, an antiangiogenic causes apparent diffusion coefficient (ADC) values to decrease, whereas a cell kill agent causes ADC values to increase. The biological effect of every therapy must be known and imaging BMs should be validated for every tumor and therapy, they recommended.
Furthermore, the value of a functional or molecular imaging technique as a BM may change depending on different features such as tumor type, anatomical location, type of therapy, biological state, and experimental set-up. An imaging technique may only be useful for a kind of tumor or therapy, or for a determinate tumor location (i.e., brain), the authors continued.
Their proposed framework or road-map for BM development includes the following:
- Multistep process: initial discovery and development, then qualification phases
- BMs should probe a disease pathway that is critical for the tumor and/or primarily affected by therapy.
- Correlating imaging changes with therapy outcomes
- Development of common measurement and analysis methods, reproducibility assessments and multicenter methodologies
"Tumor tissues exist in microenvironments, and multiple imaging tests are needed to fully assess interactions between tumor/normal tissues and changes as a result of therapies. Multiple imaging BMs and parameters are sometimes needed to characterize a tumor phenotype before and in response to therapeutic interventions," they concluded.