A group from Marseille University Hospital has provided a fresh explanation for long COVID brain fog. The team's concept is based on a visual brain pattern they discovered on patients' PET scans.
“We hypothesize that the hypometabolism pattern observed in long COVID patients with brain fog using F-18 FDG-PET might primarily be a signature of astrocyte-related glutamatergic dysregulation,” wrote lead study author Eric Guedj, MD, PhD, in an article published on 13 October in Medical Hypotheses.
Brain inflammation triggered by COVID-19 infections may disrupt how astrocyte cells regulate glutamate, a neurotransmitter involved in energy metabolism, and this malfunction ultimately leads to cognitive fatigue, the authors explained.
Moreover, the researchers proposed targeting the malfunction with therapy.
“Based on these elements, we propose that therapeutics targeting astrocytic glutamate regulation could help mitigate long-COVID neurological manifestations,” they wrote.
Long COVID is defined by the persistence or recurrence of symptoms three months after an initial infection with the SARS-CoV-2 virus, with up to 15% of patients affected, according to the authors. The condition is characterized by a lack of mental clarity, difficulty concentrating, and an inability to focus, with cognitive activities becoming effortful, they wrote.
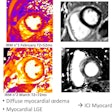
Hypometabolism detected with F-18 FDG-PET in long-COVID patients: putative astrocyte dysfunction and glutamatergic dysregulation. (A) PET scan of a healthy subject. (B) PET scan of a long COVID patient. Hypometabolic areas are indicated with arrows. (C) A diagram showing a healthy condition in which an astrocyte has a normal capacity to take up glutamate and recycle it to sustain glutamatergic neurotransmission, giving rise to normal glucose utilization and lactate production to ensure adequate neuronal energy supply. (D) A diagram showing a long COVID condition in which an astrocyte has reduced glutamate uptake capacity, leading to reduced glutamate recycling, lower glucose utilization (observed with F-18 FDG-PET as hypometabolism) and lower lactate production, dysregulating glutamatergic neurotransmission, and possibly endangering neuronal survival.Image courtesy of Medical Hypotheses.
In a previous study, Guedj and colleagues identified a pattern of hypometabolism on patient FDG-PET brain images associated with long COVID. In this article, they evaluated empirical evidence on cellular mechanisms potentially responsible for the pattern.
For example, in a recent MR spectroscopy study, a link between cognitive fatigue and glutamate dysregulation was offered to explain why cognitive control is harder to mobilize in patients after a strenuous workday, the authors wrote.
In several other cited studies, they noted that brain fog in COVID-19 patients seems regulated by similar mechanisms as those responsible for “chemo-fog” in patients with cancer. Furthermore, fatigue has previously been linked to cognitive impairment in patients with Parkinson’s disease via a similar brain network as that involved in long COVID, they wrote.
Potential therapeutic options could include ways to target the hypometabolism pattern revealed by the brain PET scans, the researchers wrote.
For example, in the interictal state of focal epilepsy, which is associated with cognitive deficits, hypometabolism is reversible after antiepileptic treatment, they wrote. Moreover, they cited a recent study that used a combination of guanfacine and N-acetylcysteine to improve cognitive function in eight of 12 long-COVID patients with brain fog.
Ultimately, further research is needed to establish a definitive link between reactive astrocytes and long COVID.
Specifically, the authors suggested additional PET imaging studies in patients with the use of translocator protein radiotracer. This tracer was used in a recent study as an index of microglial activation and revealed widespread longitudinal neuroinflammation in SARS-CoV-2-infected rhesus macaques, they wrote.
“Additional studies utilizing more specific markers or techniques targeting astrocyte function and glutamate homeostasis will be necessary for a comprehensive understanding of the underlying mechanisms,” Guedj and colleagues concluded.
The full article is available here.