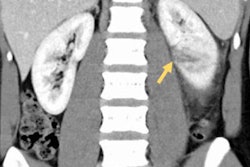
Low-cost, 3D-printed kidney phantoms provide shape-specific details that improve the quantification of SPECT/CT scans, according to a study from Würzburg, Germany, published online in the Journal of Nuclear Medicine.
The researchers demonstrated the viability of producing a two-compartment kidney model using 3D printing. They then used the model to identify the optimal SPECT/CT postreconstruction method for determining the activity of a lutetium-177 (Lu-177) radiopharmaceutical in a kidney, performing calculations with dimensions taken from CT scans of the kidney.
Augmenting SPECT/CT quantification is just one of the many potential applications of the rapidly growing technology of 3D printing in nuclear medicine, Johannes Tran-Gia, PhD, from the University of Würzburg told AuntMinnie.com via email.
"This study represents another step toward a standardized quantification of SPECT/CT for dosimetry calculations, enabling an individualized dosimetry and, therefore, improving the therapeutic success of molecular radiotherapies," he said.
Cost-effective, shape-specific
The relatively low image resolution of small objects captured on SPECT is notorious for containing hot spots -- blurry regions of high tracer activity -- that appear as if they are spilling out of the area of interest, according to the researchers. Various partial volume correction (PVC) methods assuming distinct criteria can help clarify the actual activity concentrations of tracers such as Lu-177, but exact measurements remain elusive.
 Johannes Tran-Gia, PhD, from the University of Würzburg.
Johannes Tran-Gia, PhD, from the University of Würzburg."Although many efforts have been made to improve the quantitative accuracy [of SPECT], it still represents a major source of error in internal dosimetry calculations," Tran-Gia said.
One practical correction approach involves using CT scans of physical phantoms to evaluate the ratio of observed to true activity, also known as the recovery coefficient of an object. Because industrial manufacturing of such phantoms is expensive and only profitable for production in large quantities, only a meager assortment of generic phantoms -- typically in the shape of a sphere or cylinder -- is commercially available.
Prior research by Tran-Gia et al showed 3D printing to be a more cost-effective and geometry-specific alternative to standard phantoms when applying CT scans of the kidney for partial volume correction. The researchers suggested that creating 3D-printed kidneys to bolster SPECT/CT quantification would cost as little as $2 per printed phantom.
In the current study, Tran-Gia and co-author Michael Lassmann, PhD, used a fused deposition-modeling 3D printer (Renkforce RF1000, Conrad Electronic SE) to create a generic sphere and an ellipsoid, as well as a kidney model with cortical and medullary compartments printed in four separate parts (JNM, November 2, 2017). The kidney proportions came from previously acquired sonography data from 307 males in a study by Emamian et al.
The duo filled the sphere, ellipsoid, and kidney cortex -- all of equal volumes -- with Lu-177. They scanned the models using a clinical SPECT/CT system (Symbia Intevo Bold, Siemens Healthineers), followed by a low-dose CT scan to correct for attenuation, and reconstructed the resulting scans using two different software applications (xSPECT Quant and Flash3D, Siemens).
Best volume correction method
After performing an initial volume-of-interest (VOI) analysis on the reconstructed scans, the researchers applied five different partial volume correction methods to determine the optimal form of quantifying Lu-177 activity on SPECT/CT scans of the kidney:
- Using a recovery coefficient based on figures from the computer-aided design (CAD) used to create the 3D-printed kidney
- Using a recovery coefficient based on the 3D analysis of CT scans of a kidney
- Extending the volume of interest beyond the measured contour of the kidney cortex to account for spill-out
- Assuming constant peak Lu-177 concentration throughout the kidney
- Fixing the activity threshold to 42% of the maximum voxel value covering the entire kidney and all potentially spilled-out counts
Among the five correction approaches, applying geometry-specific recovery coefficients based on the CAD model or CT scans resulted in the most accurate calculation of tracer activity, according to the researchers. The other techniques led to considerably greater errors in measuring activity.
Whereas the average difference in standard reconstruction recovery coefficients between the sphere and ellipsoid was less than 1%, the average difference was approximately 31% between the sphere-like objects and the 3D-printed kidney cortex.
| Lu-177 SPECT/CT recovery coefficients based on different methods | |||
| Correction method | Sphere | Ellipsoid | Kidney cortex |
| CAD | 0.716 | 0.711 | 0.441 |
| CT | 0.728 | 0.721 | 0.471 |
The marked difference between the recovery coefficients of a 3D-printed kidney versus a noncustomized shape suggests the importance of taking into account the geometry of the kidney for postreconstruction SPECT/CT quantification, the researchers noted. Taking into account the five partial volume correction methods, the most optimal setup to determine tracer activity is an unsmoothed SPECT/CT reconstruction combined with a recovery coefficient calculated based on CT scans of the kidney.
The relatively low cost of 3D-printed kidneys, along with their capacity to enhance SPECT/CT quantification, exemplifies the feasibility of integrating 3D printing into nuclear medicine, Tran-Gia said.
"We plan on expanding this research by manufacturing more patient-specific phantoms, e.g., by extracting anatomic parts such as the kidney from morphological patient data obtained from imaging modalities such as CT or MRI," he said. "Moreover, we will extend our efforts in optimizing partial volume corrections."