There is no evidence that gadolinium remaining in the body after MRI contrast administration has any negative health effects, according to a review published on 22 May by the U.S. Food and Drug Administration (FDA). The announcement is unlikely to settle the debate over gadolinium contrast safety, however.
In a safety announcement, the FDA stated that its review had identified "no harmful effects to date" from brain retention of gadolinium-based contrast agents (GBCAs). The agency, therefore, concluded that it was not necessary to place any new restrictions on gadolinium contrast products.
The FDA's move puts the agency at odds the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA), which in March recommended that four linear gadolinium contrast products be pulled off the market. The FDA acknowledged that its review found that linear gadolinium agents were responsible for depositing more gadolinium than macrocyclic agents, but said that no health effects were associated with either class of agents.
Disturbing findings
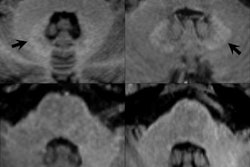
The 22 May announcement was the result of a series of disturbing findings that surfaced starting in 2013 indicating that traces of gadolinium were detectable in the brains of patients who had received MRI contrast, in some cases years after the scans occurred. The findings prompted the FDA to begin a review of gadolinium contrast safety starting in 2015.
Gadolinium is a heavy metal that in its free form is toxic to humans. However, MRI contrast agents link (or chelate) gadolinium to a carrier molecule that enables the compound to be eliminated through the kidneys after contrast administration. Gadolinium retention is known to occur with all agents, but the mechanism for this retention is still unknown, and is believed to be different for different agents.
Much of the focus on gadolinium safety has focused on the difference between GBCA products based on the type of chelate, namely linear or macrocyclic. The bonds of linear agents are believed to be more susceptible to breaking, and several studies have identified higher levels of gadolinium retention in patients who received linear contrast. On the other hand, there is much less evidence linking macrocyclic gadolinium contrast to retention.
The FDA reviewed both scientific publications and adverse event reports submitted to the agency. Both human and animal studies were reviewed, some of which tracked GBCA use for more than a year, the agency noted.
"These publications and reports show that gadolinium is retained in organs such as the brains, bones, and skin," the FDA wrote. "The publications show that linear GBCAs retain more gadolinium in the brain than macrocyclic GBCAs. However, our review did not identify adverse health effects related to this brain retention."
In addition, the FDA stated that nephrogenic systemic fibrosis (NSF) is the only known adverse health effect related to gadolinium retention. This condition is rare and occurs in a small group of patients with pre-existing kidney failure. Occurrence of NSF has dropped dramatically in recent years thanks to more effective patient screening procedures designed to prevent at-risk patients from receiving gadolinium contrast.
The FDA review noted that the PRAC's March recommendation also concluded that it could find no evidence of a health effect from gadolinium retention, but the committee still recommended pulling four linear gadolinium agents off the European market. PRAC said at the time that it was making the move out of an abundance of caution. The PRAC's decision is currently under appeal.
The next question is whether the FDA will require manufacturers of gadolinium contrast to change the labeling information for their products. The agency noted that one company, Guerbet, has changed the label for its Optimark linear GBCA by adding information about retention in various organs such as the brain and skin.
"We are reviewing the labels of other GBCAs to determine if changes are needed," the agency said.
What should healthcare providers do when faced with a decision on whether to administer gadolinium contrast? The FDA said its recommendations remain unchanged from its July 2015 update -- healthcare professionals should limit GBCA use to those circumstances when the additional information provided by the contrast agent is necessary, and they should also assess the necessity of repeat MRI scans with gadolinium contrast. Patients, parents, and caregivers should talk to their healthcare providers if they have any questions about gadolinium MRI contrast, the agency noted. The full text of the update is available on the FDA website.
Rational yet careful approach
The FDA's approach to gadolinium has received the support of at least one gadolinium safety expert, Dr. Emanuel Kanal, director of MR services at the University of Pittsburgh Medical Center in Pennsylvania, U.S. Kanal noted that research is still being accumulated about the effects of gadolinium retention, and in particular about whether there is a difference between various commercially available products -- even between different macrocyclic agents. Some of this knowledge is forcing experts to revise beliefs held just a few short years ago.
"The FDA seems to have quite intentionally selected a pathway of assuming a cautious attitude while refusing to jump -- or be pushed -- to premature conclusions without sufficiently sound scientific bases," Kanal wrote in an email to AuntMinnie.com. "In that regard, I strongly concur and applaud them for this rational yet careful approach."