Today, MRI is an integral part of the diagnostic arsenal for detecting breast pathologies. Its contribution to conventional mammographic/ultrasound work-up lies in both its sensitivity in detecting invasive or in situ carcinomas, and its excellent negative predictive value.
In clinical practice, the existence of false positives and masking matrix enhancement effects often complicate interpretation and can necessitate supplementary examinations such as targeted ultrasound, biopsies with clip placement, and MRI clip-monitoring sequences.
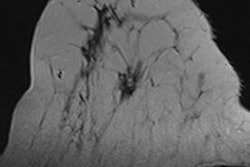
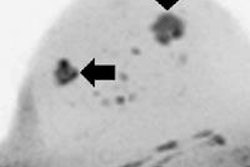
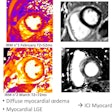
First MRI screening of a 37-year-old patient, carrier of the gene mutation BRCA 1 during the 22nd day of her cycle. An asymmetrical background matrix enhancement effect potentially masking a, b, c. Close monitoring on the 10th cycle day (d) allows validation of the classification BI-RADS 2. Image courtesy of JFR.
Contrast uptake without conclusive mammographic or echographic interpretation necessitates iterative surveillance or MRI-guided biopsy, which can be difficult to undertake. We will outline several key points for optimal interpretation of breast MRI that depend on rigorous management of all stages of the exam: indications, patient preparation, interpretation, and result management.
In France, the prerequisite for any breast MRI is respect for the indications validated by the National Health Authority (Haute Autorité de Santé) and the European Society of Breast Cancer Specialists (EUSOMA) in 2010.
MRI must not replace an incomplete conventional assessment or an American College of Radiology (ACR) 4 or ACR 5 category lesion biopsy. On the contrary, it is indicated when the sensitivity benefits brought by MRI to the conventional assessment are potentially higher than the negative effects from false positives: in addition to the full noncontributory standard assessment (ACR 0, Paget's disease, and metastatic axillary lymph nodes detected with mammography-ultrasound ACR 2) in the screening of women with high genetic risk, in the extended assessment of certain breast cancers when there is a significant risk of multifocality or multicentricity that could entail a modification of therapeutic management (lobular histology, women under 40, discrepancy of radioclinical lesion size), or before specific management (neoadjuvant chemotherapy or preoperative radiotherapy).
In indications for an extended assessment of a known cancer, a multidisciplinary discussion of the case allows the team to validate the utility of MRI, which must not delay therapy.
Patient management
Information provided by the patients is essential and allows us to obtain their cooperation and also ensures adherence to further exams or complementary biopsies. Other than indications for an extended assessment of proven cancer where delay to therapy start must be taken into account, MRI must be scheduled where physiological matrix enhancement effect is minimal, either in the second week of the menstrual cycle for premenopausal patients or after stopping thyroid-stimulating hormones.
An essential factor is good positioning of the patient, which obviates skin folds responsible for artifacts, optimizes topographic correlation with conventional imaging, and allows the reproducibility of a comparison if follow-up is necessary. The exam protocol associates T1 and T2 sequences without fat saturation, and a dynamic sequence performed after injection of gadolinium chelate at a dose of 0.1 to 0.2 mmol/kg with the aid of an automatic injector at a flow rate of 2 to 3 ml/sec followed by 20 cc of physiological serum.
The goal of these T1 and T2 sequences is to search for benign signs (liquid, fat) and to analyze lesion contours, which allows differentiation of mass and nonmass lesions. Slice thickness must be as low as possible.
Treatment of images by subtraction allows higher sensitivity in the detection of contrast uptake. The dynamic protocol is a compromise between temporal and spatial resolution. For optimal temporal resolution, sequences should be short (less than 2 minutes) repeated during 7 minutes 30 seconds (malignant lesions most often display intense and early kinetic pattern -- maximum specificity at 90 seconds to 2 minutes -- with late wash-out). Optimal temporal resolution can be obtained by isometric and millimetric 3D sequences.
Knowledge of the clinical context and factors that could produce matrix enhancement effects is indispensable for a relevant interpretation. Breast MRI is always performed together with, or as a complement to, conventional imaging. It must always be interpreted with knowledge of the clinical, mammographic, and ultrasound assessment. When screening, comparison with earlier assessments must be systematic. It can be useful to compare the entire range of earlier assessments. An optimal morphological analysis necessitates the reading of images in three planes (lesion edges, distribution) and can be improved by reading native sequences, before and soon after injection, which offers a better spatial resolution than subtraction.
Analysis in three spatial planes allows us to give an accurate topography for each MRI lesion, prime factor for correlation with conventional imaging. When interpreting MRI, radiologists must use international BI-RADS (ACR 2013) classification, the goal of which is to harmonize reports and the next course of action.
Effective use of MRI results
Results from the three modalities of MRI, mammography, and ultrasound must be synthesized and conclude with a clear course of action to follow: close surveillance or biopsy, the methods for which must be specified (ultrasound-, MRI- or stereotaxy-guided, in the absence of conclusive mammographic or ultrasound interpretation).
The MR image must not be classed BI-RADS 0 except for in the specific case of a provisional report while waiting for earlier documents. With an MRI contrast uptake ACR 4 or 5, one must first re-read the mammography assessment. Targeted ultrasound involves a learning curve and must be performed when specialists are well-versed in MRI (exact topography of contrast uptake, and specifically, location within the mammary gland and in relation to the fat).
Management of BI-RADS 3 lesions is not consensual and depends on indication. To reduce false positives and BI-RADS 3 due to matrix enhancement effects, a double reading of benign MRIs could be organized. EUSOMA advocates a yearly scan rate superior to 150 breast MRI/year. Finally, the possibility of recourse to MRI-guided biopsy and to a multidisciplinary breast cancer focus meeting should be formalized.
Dr. Isabelle Doutriaux is from the department of medical imaging at René Gauducheau Center, in Nantes, France.
Editor's note: This is an edited translation of an article published in French on 19 October 2015 by le Quotidien des JFR (Journées Françaises de Radiologie Diagnostique et Interventionnelle), the daily newspaper of the French national congress of radiology. A scientific session on breast MRI was held on the afternoon of 19 October. Translation by Frances Rylands-Monk.