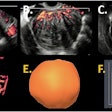
There is a compelling need to determine the relationship between the use of assisted reproductive technologies (ART) and breast cancer risk, particularly because studies have so far produced conflicting findings, according to research presented at the recent EUSOBI annual meeting in Aberdeen, U.K.
The American Society for Reproductive Medicine released advice in 2024, "Fertility Drugs and Cancer." It stated that there does not appear to be an increased risk of breast cancer associated with ART, but there is a clear lack of information from breast radiologists in the literature, noted Dr. Micòl Bottalico of the Department of Diagnostic Imaging, Oncological Radiotherapy, and Hematology at Policlinico Agostino Gemelli in Rome, and colleagues.
With the increased use of ART (approximately 7.4 million U.S. women used infertility treatments between 2004 and 2010, the research team notes), the need to establish radiological surveillance guidance for women exposed to these treatments should be prioritized, the authors wrote in an e-poster.
The team systematically reviewed the literature from 1999 to the present, dividing the studies into three categories based on the type of infertility drug examined in the study: clomiphene citrate (9 studies), gonadotropins (6 studies), and progesterone (4 studies). It then examined the associations reported between breast cancer risk and drug exposure for each category, assessing the hazard ratio (HR) and standardized incidence ratio (SIR) for cancer occurrence in individual patient groups. The aim was to establish the epidemiological association of the drugs with breast cancer risk.
The researchers pointed out that several studies have established an increase in breast cancer risk in women given gonadotropins, but one large-scale study found “no significant overall increase” in breast cancer risk in women treated with gonadotropins. However, the same study found a “slightly higher” risk for women undergoing multiple cycles of ART with gonadotropins -- a finding echoed in other studies, some of which also noted increased risk for women with prolonged infertility.
Research involving the use of clomiphene citrate has yielded similar results, Bottalico and colleagues continued. Three studies -- one large-scale -- showed an increased incidence of breast cancer in women who were given clomiphene citrate, although the finding was variable, from nonsignificant to significant, and in two cases. Subgroups were specified in these findings (i.e., “in women who had used this drug and experienced a delay of more than 12 months before conceiving” and “associated with hormonal infertility and with a cumulative dose of 2,000 mg”).
The authors caution that some studies reviewed involved a small number of cases or had other limitations, such as incomplete documentation of dosages, varied follow-up, heterogeneous study groups, or insufficient control for other risk factors. “The risk was higher for [breast cancer] in patients treated with only one or two treatment cycles, suggesting that infertility itself plays a potential role in increasing the risk of [breast cancer].”
The evidence for increased risk of breast cancer with the use of ART is unclear, the authors concluded. They call for standardized radiological surveillance protocols to be developed for women undergoing these fertility treatments in light of these findings, especially for women with additional risk factors, such as family history or long-term infertility.
Read the EUSOBI e-poster here.