Interventional technology developer BTG Intervention Medicine is highlighting a pair of clinical studies presented at the recent Cardiovascular and Interventional Radiological Society of Europe (CIRSE 2015) meeting in Lisbon, Portugal.
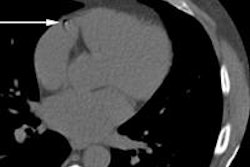
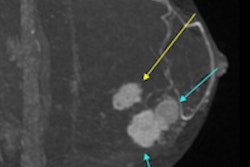
Preliminary study results support the efficacy and tolerability of BTG's drug-eluting DC BeadM1 bead for treating liver-confined hepatocellular carcinoma (HCC), the company said. The results showed that patients had achieved a median overall survival of 41 months from the use of the doxorubicin-eluting bead, according to BTG.
In addition, the team led by Dr. Camillo Aliberti from the Istituto Oncologico Veneto in Padua, Italy, reported that there was a median progression-free survival of 15 months with no significant treatment-related adverse events. The single-center, retrospective, single-arm, open-label study had reviewed the treatment of 547 HCC patients treated with DC BeadM1.
In another study presented at CIRSE 2015, BTG's TheraSphere yttrium-90 (Y-90) glass microsphere treatment for advanced HCC was found to yield a significantly lower total lifetime cost of treatment compared with sorafenib (21,441 pounds versus 34,050 pounds), thanks to improvements across time to progression (6.2 versus 4.9 months), increased median overall survival (13.8 versus 9.7 months), and increased quality-adjusted life years (1.12 versus 0.85 years).