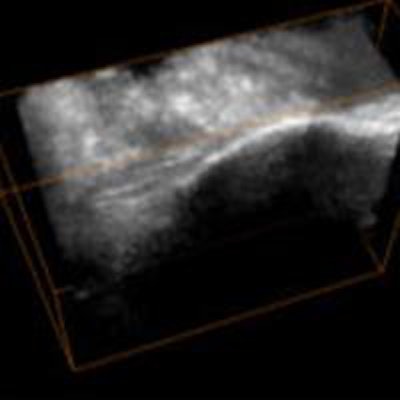
Researchers are closing in on a radiation-free alternative to intraoperative fluoroscopy in orthopedic surgery. A newly validated algorithm delivers clear bone surface images in near real-time from ultrasound images, according to a study in the International Journal of Computer Assisted Radiology and Surgery.
The novel segmentation technique is based on multiresolution analysis of images to localize bone surfaces in 3D ultrasound volumes. The study team validated the technique in scans obtained from 29 trauma patients with distal radius and pelvic ring fractures. The next step will be to apply the technique intraoperatively, rather than in the clinical setting, where images for the study originated (IJCARS, July 2015, Vol. 10:7, pp. 1279-1287).
How does it work? "Basically you collect the ultrasound images and the algorithm will extract only the bone surfaces from that scan -- you don't need the soft tissues," said lead author Ilker Hacihaliloglu, PhD, in an interview with AuntMinnieEurope.com. "Bone surgeons then look at those bone surfaces to see where the fracture is."
Fluoro's flaws
The most common intraoperative imaging modality in orthopedic surgery is 2D fluoroscopy, used to identify bone fragments, visualize bone reductions, and guide surgical tools. But 2D fluoro is inadequate in a 3D surgical environment, and, as he recalled, surgeons at Vancouver General Hospital were dissatisfied with it.
 Ilker Hacihaliloglu, PhD, from Rutgers University.
Ilker Hacihaliloglu, PhD, from Rutgers University."They were having problems because the anatomy they were working with was 3D, so they were basically trying to solve a 3D problem using 2D images," noted Hacihaliloglu, professor of biomechanical engineering and director of the computer-assisted surgery and therapy laboratory at Rutgers University in NJ. "The second problem was the radiation exposure of x-ray fluoroscopy images."
As solutions go, ultrasound is cheap, though it has a couple of drawbacks that a new technique would need to solve for intraoperative imaging.
B-mode ultrasound images are characterized by high levels of noise and reverberation artifacts, image quality is user-dependent, and bone surfaces are blurred, which makes it difficult to both interpret images and to use them as a basis for navigated interventions, according to Hacihaliloglu and colleagues from Rutgers and the University of British Columbia in Vancouver.
For ultrasound to become a practical modality for orthopedic interventions, bone surfaces will have to be extracted quickly, accurately, and automatically from ultrasound scans in the clinical setting, the team wrote.
Previous algorithms based on intensity and local gradient image information have been reported, but they are usually limited to 2D and are highly sensitive to variations in data and imaging parameters.
Phase-based analysis
The approach in this study, based on phase information in B-mode ultrasound, had previously been used to enhance soft-tissue interfaces in ultrasound data. The group's earlier work translated 2D phase-based images to 3D, and established that phantom-based bone surfaces can be localized with submillimeter accuracy using ultrasound. That work led to the present phase-based algorithm, which uses Log-Gabor filters to extract local phase information from ultrasound images.
The purpose of this study was to determine whether the imaging processing tool would enable accurate and rapid extraction of bone surfaces in 3D ultrasound volumes, testing it on pelvic fractures.
The study looked at 29 patients presenting with a pelvic radial fracture or pelvic ring injury to Vancouver General Hospital's level 1 trauma facility between 2010 and 2013.
All patients underwent preoperative CT along with ultrasound on a GE Voluson scanner (GE Healthcare) equipped with a 3D RSP5-12 transducer, a mechanized probe in which a linear-array transducer sweeps through an arc of 20°. Preoperative CT images served as the reference standard.
The algorithm extracted bone surfaces from the ultrasound volumes using the image phase-based processing method. The filter parameters of the algorithm were automatically optimized via the validated framework.
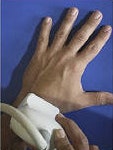
Radius images included dorsal, volar, and radial views of the injury. Pelvic ring images were acquired at the crest/iliac fossa. Bilateral scans were acquired for patients under anesthetic.
Following the extraction of bone surfaces from both CT and ultrasound, the investigators refined their initial landmark-based alignment using a surface-based registration algorithm, with point sets represented as Gaussian Mixture Models (GMMs).
An expectation maximization algorithm is used to solve the registration between CT and ultrasound, with the centroids of the GMMs belonging to the ultrasound point set that had been transformed to fit the CT GMMs, the team explained.
To compare the CT gold standard with the 3D phase-based technique, the team created a signed distance map around the 3D bone surface, and each value in the phase-processed image was mapped to its corresponding location in CT.
Minimal error
Among 29 patients, the results showed four with distal fractures and 25 with pelvic ring injuries; two patients were excluded from analysis because the CT scan was not received.
- The initial anatomical landmark registration error was a mean 32 mm for pelvic patients and 0.27 mm for distal radius cases.
- The mean surface fit error was 0.62 mm for pelvic ring injury patients and 0.21 mm for distal radius patients.
Five of the 13 pelvic cases obtained in the ward had markedly larger mean errors compared with the other 18 scans, reaching 1.25 mm.
"On average, we found submillimetric surface fitting errors between our ultrasound-derived surfaces and the 'gold standard' surfaces derived from the CT scans," the authors reported.
In five of the 23 pelvic fracture cases, the extracted surfaces could be used to assess the fracture, and in all cases the extracted surfaces could be used to evaluate the fracture location, they added.
The study did not establish that the method could be implemented in real-time, but the project to complete this step is underway, though there are challenges, Hacihaliloglu said. Ultrasound is a user-dependent modality, so images can be "really good or really bad," depending on who acquires them and how, and as a result a learning curve will be an unavoidable part of image optimization. Also, when the project was first completed, the algorithm still had some lag time before the images could be visualized, but the process has now evolved far enough to completely eliminate that worry, he explained.
Other than that, it's basically a task of finding high-impact regions on which to make best use of the technique, and the group has started working on a couple of good candidates for that job as well, according to Hacihaliloglu.
"One project is for spinal surgery, we're going to use both ultrasound and preoperative CT scans," he said. "Another is imaging scoliosis, which is currently monitored using x-ray," with potential long-term impact on young patients from about 10 to 13 years old who are scanned frequently with radiation-bearing modalities to monitor the condition.
"There are lots of things you can do," Hacihaliloglu concluded. "It's a cheap imaging modality as well. With MRI you need to schedule a scan and it costs a lot of money, but most of us have an ultrasound machine. If you get an idea, you can try it out."