A new method of soft-tissue imaging could enable live tracking of respiratory treatments in cystic fibrosis patients, reports an international research team. The technique -- which measures the refraction of a grid pattern of x-rays passing through the lungs -- has been successfully demonstrated in live mice, and may eventually find application in visualizing other soft tissues, such as the brain and heart.
Cystic fibrosis is a life-threatening genetic disorder that affects the exocrine glands, resulting in unusually thick secretions. In lungs, mucus is supposed to keep the airways moist, along with forming a conveyor belt, moved by beating cilia, which carries away foreign particles and pathogens. In cystic fibrosis patients, however, the reduced, thicker mucus flows less easily -- resulting in a buildup that can cause inflammation, breathing difficulties, and increased susceptibility to bacterial infection.
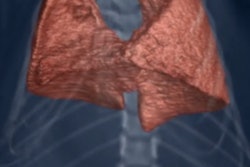
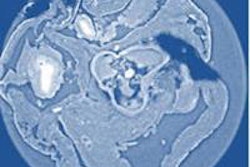
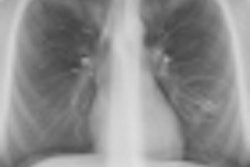
 Researchers at Monash University are developing high-resolution phase contrast x-ray imaging for in vivo observation of the airway surface. All images courtesy of Kaye Morgan.
Researchers at Monash University are developing high-resolution phase contrast x-ray imaging for in vivo observation of the airway surface. All images courtesy of Kaye Morgan.Respiratory therapies for cystic fibrosis patients typically focus on increasing hydration of the airways, thereby helping to improve mucus flow. Tracking the progress of these treatments, however, is challenging. "At the moment, we typically need to wait for a cystic fibrosis treatment to have an effect on lung health, measured by either a lung CT scan or breath measurement, to see how effective it is," explained lead author Kaye Morgan, PhD, a research fellow from Monash University in Australia. With successful medications often taking months to have a measurable impact, progress in developing new treatments is correspondingly slow (American Journal of Respiratory and Critical Care Medicine, 15 August 2014, Vol. 190:4, pp. 469-472).
The challenge lies in imaging the surface layers of liquid in the airways. These are usually only a few tens of microns across, bear a close resemblance to the underlying tissue, and -- given the passage of air in and out of lungs -- constantly move around. Consequently, any technique for imaging this interface needs to be high-resolution, as well as sufficiently fast and sensitive.
Morgan and colleagues have developed an imaging method they call single-grid-based phase contrast x-ray imaging. Unlike conventional radiography, which measures the absorption of x-rays, the new approach measures the refraction of a grid pattern of radiation as it passes through the soft tissues.
"A good analogy is the patterns we see on the bottom of swimming pools," explained Morgan. At the detector, the x-ray grid will appear distorted in accordance with the properties of the tissues that the rays have passed through -- much in the same way that tiles in a pool appear distorted when seen through water. "By tracking the distortions in the grid pattern, we can reconstruct the airway structures."
In vivo imaging
To test their method, the researchers imaged the airways of eight anesthetized mice. Using a nebulizer, each mouse was treated first with a saline control solution, and then with a treatment designed to block the dehydrating effect of the cells lining the airway. X-rays from a synchrotron traveled through a 25.4 micron grid to create the desired pattern; this produced images at the detector with 0.18 micron-sized pixels. Images were recorded at three-minute intervals for 15 minutes after each treatment.
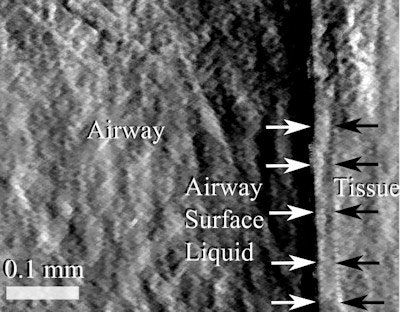 Single-grid-based phase contrast x-ray imaging can visualize the airway, surface liquids, and underlying tissues in mice.
Single-grid-based phase contrast x-ray imaging can visualize the airway, surface liquids, and underlying tissues in mice.The method successfully imaged the airway, surface liquids, and underlying tissues. A noticeable increase in the surface hydration depth was observed after treatment in comparison with the control. "The new imaging method allows us for the first time to noninvasively see how the treatment is working, 'live' on the airway surface," Morgan said.
"This is a novel and interesting biomedical application," said Mark Anastasio, a biomedical engineer from Washington University in St. Louis. With existing solutions unable to reveal such subtle soft-tissue interfaces, he adds, this result "motivates the further development of x-ray phase-contrast imaging technologies."
"Moving from ex vivo to in vivo is a huge step along the course of applying phase contrast x-ray projection imaging to medical imaging," agreed Ke Li, a medical physicist from the University of Wisconsin-Madison. However, Li questions the practicality of using a synchrotron x-ray source in a clinical environment, especially given the high radiation dose necessary for such an ultrafine pixel size.
Some of these concerns may soon be addressed, with Morgan and colleagues now exploring how their work might translate into a clinical setting. At the same time, the team is investigating other possible medical applications, looking both at lungs and other soft-tissues, such as the brain and heart.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.