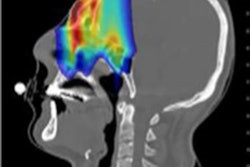
Exploiting the superior dose deposition of particle therapy for clinical benefit necessitates an accurate technique for verifying dose delivery. Because the treatment beam stops inside the patient, such monitoring requires detection of secondary radiation -- such as prompt gamma rays or positron annihilation photons -- created when the incident beam interacts with the tissue.
The only clinical application of such approaches is PET imaging of annihilation photons -- which is employed in just a few hadron therapy facilities. Currently, however, all these facilities perform PET after treatment delivery, and only detect positron emitters with half-lives (T1/2) of between 2 and 20 minutes (such as O-15, C-11, P-30, and K-38g). As a result, image acquisitions of at least a few minutes are needed and information is delayed with respect to dose delivery. This delay also increases biological washout, thereby reducing the PET activity.
In contrast, PET during treatment would enable imaging of short-lived (T1/2 below 19 s) nuclides. Such "beam-on PET" could provide real-time feedback with less biological washout. "The main benefit of using short-lived rather than long-lived nuclides is that potentially one obtains much faster feedback and can thus interrupt a 'wrong' irradiation before it is finished," explained Peter Dendooven, from the University of Groningen's KVI-Center for Advanced Radiation Technology.
To assess the feasibility of beam-on PET, Dendooven and colleagues measured the production of positron emitters during the stopping of 55 MeV protons. The aim: to identify which short-lived positron emitters are created in sufficient quantities to be relevant for in vivo verification of proton dose delivery (Physics in Medicine and Biology, 5 November 2015, Vol.60:23, pp. 8923-8947).
What is emitted?
The researchers irradiated graphite, water, phosphorus, and calcium targets with 55 MeV protons and measured the intensity of the 511 keV positron annihilation photons. The different nuclides were identified based on their half-life, by fitting the 511 keV intensity decay with one or more exponential decays and, where relevant, a constant background. To avoid the large prompt gamma background seen when the beam is on, they used a pulsed proton beam and only analysed beam-off periods.
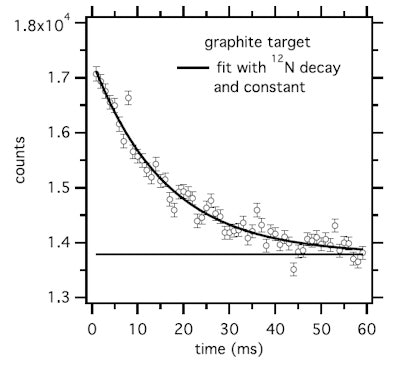 Time spectrum of the 511 keV intensity during the beam-off period for a graphite target and 60 msec on/off beam pulsing. The result of a fit considering a constant plus the decay of N-12 is shown.
Time spectrum of the 511 keV intensity during the beam-off period for a graphite target and 60 msec on/off beam pulsing. The result of a fit considering a constant plus the decay of N-12 is shown.For the water target, the researchers examined the production of N-12 (T1/2 = 11 ms) and O-13 (T1/2 = 8.58 ms). Neither was definitely observed, and they concluded that no short-lived nuclides are produced on oxygen. For graphite targets, the most copiously produced short-lived nuclide was N-12, which was produced at 9% of the rate of C-11 (T1/2 = 1223 s) on carbon. The 511 keV intensity-time spectrum recorded with 60 ms on/off beam pulsing fitted well to the N-12 decay plus a constant.
For the phosphorus target, the most prevalent short-lived nuclide was P-29 (T1/2 = 4.1 s), which was produced at 20% of the rate of P-30 (T1/2 = 150 s). For the calcium target, K-38m (T1/2 = 0.92 s) was the most copiously produced, at 113% the rate of K-38g (T1/2 = 7.6 m). The spectrum was best fit with K-38g, K-38m, and K-37 (T1/2 = 1.22 s), implying that all three are produced in relevant quantities.
Dendooven and colleagues used these measured production rates to determine the production of short-lived nuclides in PMMA and representative tissues: carbon-rich (adipose) and oxygen-rich (skeletal muscle) soft tissue, plus compact and cortical bone. Considering a continuous proton beam delivered by a cyclotron, they calculated the number of decays integrated from the start of a 55 MeV proton irradiation.
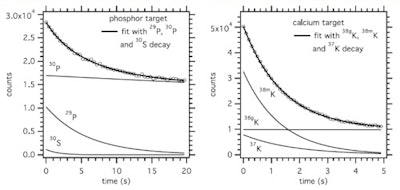 Time spectrum of the 511 keV intensity during the beam-off period for a phosphorus target and 20 sec on/off beam pulsing (left), and a calcium target and 5 sec on/off beam pulsing (right). The results of the fit considering the decay of the indicated nuclides is shown, as well as the individual contributions as deduced from the fit (thin lines).
Time spectrum of the 511 keV intensity during the beam-off period for a phosphorus target and 20 sec on/off beam pulsing (left), and a calcium target and 5 sec on/off beam pulsing (right). The results of the fit considering the decay of the indicated nuclides is shown, as well as the individual contributions as deduced from the fit (thin lines).In carbon-rich materials, the number of PET decays from N-12 dominated for irradiation times of up to 70 sec in adipose and 45 sec in PMMA. For bone, O-15 dominated, except for irradiations below about 15 sec in compact bone and 8 sec in cortical bone, where N-12 was dominant. Short-lived nuclides produced on phosphorus and calcium, P-29 and K-38m, provided 2.5 times more PET counts than the long-lived P-30 and K-38g during a 70 sec irradiation (the typical time to deliver 2 Gy in a volume of 1 l), implying that bone tissue should be more visible in beam-on than postirradiation PET.
Prompt gamma comparisons
Finally, the researchers compared N-12 PET with prompt gamma imaging using a state-of-the-art knife-edge slit camera. They chose N-12 as it is produced on carbon, which is abundant throughout the body, and its very short half-life may allow response at a time scale of 50-100 ms.
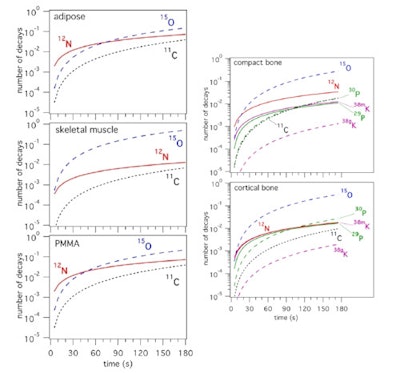 The number of decays of PET nuclides integrated from the start of an irradiation as function of time during the irradiation, for an irradiation with 55 MeV protons and scaled to a beam intensity of 1 proton per second. Short-lived nuclides are shown with solid lines, long-lived ones with dashed lines.
The number of decays of PET nuclides integrated from the start of an irradiation as function of time during the irradiation, for an irradiation with 55 MeV protons and scaled to a beam intensity of 1 proton per second. Short-lived nuclides are shown with solid lines, long-lived ones with dashed lines.They estimated that for PMMA, the number of N-12 PET counts was about 10 times larger than the prompt gamma counts, for similar image resolution. In tissue, the ratio of N-12 PET to prompt gamma counts depends upon the carbon content. For adipose, this ratio is similar to that of PMMA, while for other tissues, it lies between one and three. This demonstrates that N-12 PET imaging has the potential to provide equal or superior proton range information to that obtained via prompt gamma imaging.
The authors are now addressing the practical implementation of this approach. "We are presently working out the details of imaging the short-lived nuclides, especially N-12, such that the best dose delivery information is obtained," Dendooven explained. "This involves real-time image reconstruction and its coupling to the beam delivery time structure, and separation of the N-12-related image contribution from that of longer-lived isotopes. Better knowledge of the production rates as function of proton beam energy is also needed."