The introduction of combined PET/MRI scanners was enabled by the replacement of photomultipliers with solid-state PET detectors, such as avalanche photodiodes (APDs), which can operate inside the MRI environment. Nevertheless, simultaneous PET and MR measurements employ electronic circuits and instrumentation that can interfere in multiple ways, and in both directions.
A European research team has developed a preclinical PET/RF insert for a clinical MR scanner, using silicon photomultipliers (SiPMs). "SiPMs are a further development of APDs with a higher gain, lower temperature dependence, and faster response time," explained Bjoern Weissler, a senior scientist at Philips Research Europe and RWTH Aachen University. "The first two properties are advantageous both for PET and for PET/MR. The faster response time means that SiPMs, unlike APDs, are TOF [time-of-flight] compliant."
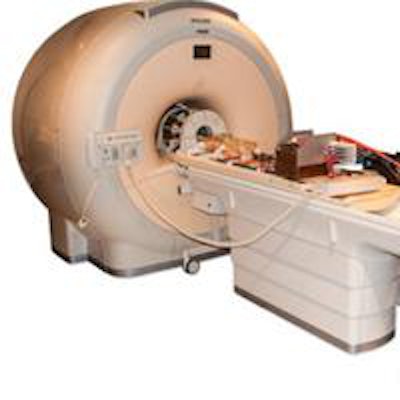
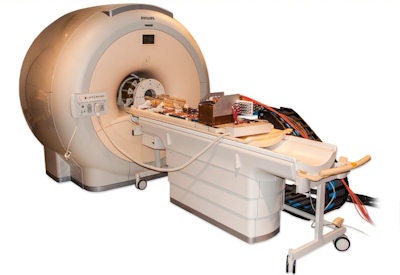 The PET/RF insert mounted on the patient table of a clinical 3-tesla MRI system.
The PET/RF insert mounted on the patient table of a clinical 3-tesla MRI system.The device was created within an EU-funded project HYPERImage, which comprised the whole imaging chain from sensor (SiPM), digitization (ASIC) up to full system design. The big challenge when creating a PET/RF insert is to develop a scalable architecture that can supply, control, cool, synchronize, and read out a high number of detector channels, while minimizing interference with MRI operation. Weissler and colleagues designed a system based on a ring of RF-shielded detection modules, each containing a 22 × 22 scintillation array of 10 mm LYSO crystals and 16 monolithic 2 × 2 SiPM arrays. To avoid PET signal degradation, the detector modules digitize the SiPM signals directly inside the MRI bore.
The PET/RF insert contains 10 of these modules in a ring around a PET-compatible MRI RF transmit/receive coil. When the insert is placed inside a clinical MR scanner, the hybrid field-of-view is 160 × 30 mm. The researchers have performed a series of interference tests on the insert to study the interactions between the two modalities (Physics in Medicine and Biology, Vol. 59:17, pp. 5119-5139).
Impact of PET on MR
The researchers first assessed the impact of the PET insert on the MR system. They evaluated the homogeneity of the static magnetic field (B0) by scanning a bottle-phantom and plotting the maximum B0 distortion in a spherical region-of-interest (ROI). The ROI with a B0 distortion of less than 2 ppm peak-to-peak had a diameter of 56 mm; standard automatic volume shimming increased this to 90 mm.
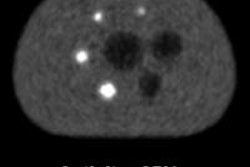
They also checked for unwanted spurious emissions from the PET detector electronics, by performing MR scans with the PET system disconnected from the power and while measuring seven Na-22 point sources. The average MR signal over the complete bandwidth was 242 ± 4 with the PET switched off and 292 ± 26 during point source measurement. This increased mean noise floor translates into a decrease in the image signal-to-noise ratio (SNR).
','dvPres', 'clsTopBtn', 'true' );" >
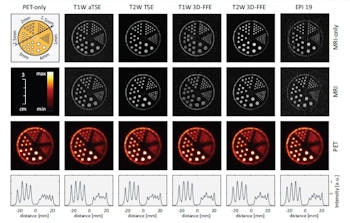
Click image to enlarge.
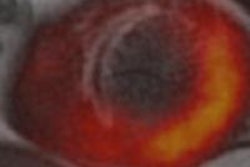
PET only (left column), MRI only (first row) and PET-MR measurements of a hot rod phantom. The orientation of the profiles through the PET images (bottom row, intensity scaled individually) is shown in the schematic of the phantom (top left).
To quantify these changes, the researchers scanned the bottle-phantom and calculated the SNR in five ROIs. For a spin echo sequence, use of PET decreased the image SNR by 14%, while image uniformity changed from 93.6% to 92.9%. Comparing MR-only and PET/MR images for six other sequences revealed a mean SNR degradation of 13% and a small change average in image uniformity of -0.6%. RF artifacts (seen as dotted lines) were only visible in sequences with very low SNR, such as some echo planar imaging (EPI) sequences.
Impact of MR on PET
Next, Weissler and colleagues performed a 23 minute PET measurement on seven Na-22 point sources, while executing three MRI sequences (turbo spin echo, fast field echo, and EPI). Gradient-intense EPI sequences led to heating of the detector electronics, but only by about 0.04 K over a three minute sequence. As SiPM gain decreases with temperature, this led to a photopeak shift of about 0.5% and a change in coincidence count rate of only about 2%.
The energy resolution (29%), time resolution (2.5 ns), and jitter of synchronization pulses detected by the ASICs (22.5 ps) appeared unaffected by the MR sequences. Five high-resolution point sources were used to determine the volumetric spatial resolution, which was lower than 1.8 mm3 in the FOV center and was unaffected by the tested MRI sequences.
Imaging performance
The team assessed the PET/MR imaging performance using a hot rod phantom filled with F-18 FDG. They recorded a reference PET scan outside the MR scanner and five scans inside, from 16.7 MBq to 8 MBq. MRI operation did not affect the spatial resolution of the PET images (1.5 mm rods were separable) and no artifacts were observed. In the MR images, ghosting artifacts were seen in about 26% of EPI scans, with 7.6% suffering from severe ghosting. All other MR images were free from PET-related artefacts or distortion.
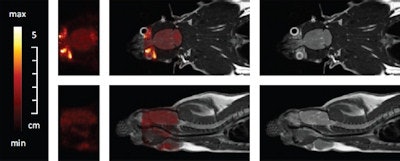 Simultaneous PET/MR measurement of a rat brain with about 5 MBq FDG. Coronal slices (top) and sagittal slices (bottom). PET only (left), fused PET/MR (middle) and MR images (right).
Simultaneous PET/MR measurement of a rat brain with about 5 MBq FDG. Coronal slices (top) and sagittal slices (bottom). PET only (left), fused PET/MR (middle) and MR images (right).Finally, the researchers performed simultaneous PET/MRI on a living rat. After anesthetization, the rat was injected with 7 MBq of FDG in the tail and a PET brain scan performed one hour later. Several MR sequences were used during the 50 minute PET scan. The resulting images confirmed the capability of simultaneous in vivo PET and MR data acquisition inside a clinical MRI scanner.
"We learned from this insert, that digitization in the MRI bore is possible and advantageous," Weissler said, adding that this early digitization makes the infrastructure suitable for clinical TOF applications. "For the preclinical insert, the detector stacks are optimized for spatial resolution by using small crystals and spreading their light over multiple SiPMs," he explained. "A TOF system for human patients would use crystals with a size close to that of the SiPMs and coupled, in the best case, directly one-to-one. We have made such detector stacks and tested them successfully in the MRI."
The team is also developing a second-generation insert using a purely digital sensor. "The next step in detector evolution is digital SiPM, which skips the analogue step completely and counts detected photons directly at the SiPM cells," Weissler told medicalphysicsweb.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.