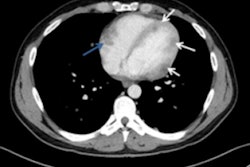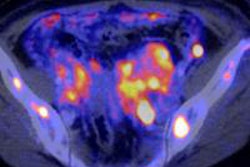
In the right hands, PET/CT has a significant clinical impact on management decisions in more than 40% of patients with recurrent stage III/IV malignant melanoma (MM) by detecting additional sites of distant metastatic disease and characterizing indeterminate CT findings, U.K. researchers have found.
"FDG PET/CT (18F-fluorodeoxyglucose positron emission tomography-CT) is an effective tool in recurrent stage III/IV malignant melanoma," noted Dr. Manil Subesinghe and colleagues from the department of nuclear medicine, Leeds Teaching Hospitals National Health Service (NHS) Trust."Effective use of FDG PET/CT is via referral from the specialist skin cancer multidisciplinary team."
In a highly specialized tertiary center, at which individual cases are discussed and patients are appropriately referred on for further evaluation, FDG PET/CT can alter or preclude potential surgical or chemotherapeutic management, they wrote in an article published online on 10 September by Insights into Imaging. A multidisciplinary approach is the standard of care for delivery of oncologic patient management throughout the U.K., and is associated with improved survival through benefits gained by specialist review of imaging, improved accuracy of staging, and resultant optimization of surgical management, they explained.
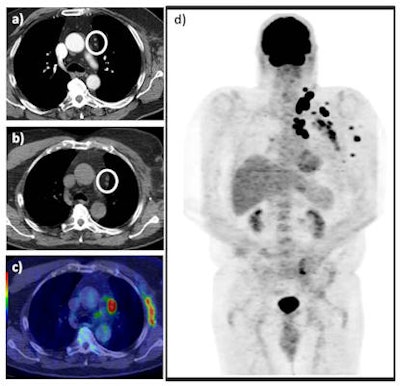 A 73-year-old man with previous recurrent stage IIIC disease was found to have an enlarged fine needle aspiration positive, left supraclavicular lymph node on clinical follow-up. A staging CT revealed presumed postsurgical changes in the left axilla and a couple of sub-5mm mediastinal lymph nodes (white circle) (a). A subsequent FDG PET/CT revealed FDG-avid left axillary soft-tissue recurrence and intensely hypermetabolic sub-cm nodal disease in the mediastinum (white circle) as seen on the axial unenhanced CT (b) and axial fused PET/CT images (c), in addition to multiple further sites of FDG-avid loco-regional disease as seen on the maximum intensity projection (d). This resulted in change from potential radical surgery to chemotherapy, which represented a major clinical impact. All images courtesy of Dr. Manil Subesinghe.
A 73-year-old man with previous recurrent stage IIIC disease was found to have an enlarged fine needle aspiration positive, left supraclavicular lymph node on clinical follow-up. A staging CT revealed presumed postsurgical changes in the left axilla and a couple of sub-5mm mediastinal lymph nodes (white circle) (a). A subsequent FDG PET/CT revealed FDG-avid left axillary soft-tissue recurrence and intensely hypermetabolic sub-cm nodal disease in the mediastinum (white circle) as seen on the axial unenhanced CT (b) and axial fused PET/CT images (c), in addition to multiple further sites of FDG-avid loco-regional disease as seen on the maximum intensity projection (d). This resulted in change from potential radical surgery to chemotherapy, which represented a major clinical impact. All images courtesy of Dr. Manil Subesinghe.The researchers studied 45 patients who were referred for further evaluation with FDG PET/CT. They compared findings on FDG PET/CT with prior contrast-enhanced CT scans, and determined the clinical impact on subsequent management decisions retrospectively. They defined major clinical impact as a change in treatment plan, while minor impact was defined as confirmation of known sites of disease as identified on prior CT.
Most CT scans were performed on a 64-slice system (Somatom Sensation; Siemens Healthcare), using a contiguous 5-mm reconstruction following a bolus of 100 mL (75 mL to 125 mL) iodinated contrast medium. The FDG PET/CT examinations performed between June 2008 and June 2010 were conducted on a 16-slice Discovery STE PET-CT system (GE Healthcare), and from June 2010 to June 2012 on a 64-slice Gemini TF64 unit (Philips Healthcare). The CT images were retrieved from and reviewed on the PACS (IMPAX, Agfa Healthcare), whilst PET/CT imaging was reviewed on a specialized PET/CT workstation (XD3, Mirada-Medical).
A total of 51 FDG PET/CT examinations on 45 patients met the study's criteria. The procedure had a major clinical impact in 21 cases (41.2%), of which 18 examinations were performed in patients with proven or suspected stage IV MM. FDG PET/CT had a minor impact in 23 cases (45.1%), and there were five false-positive cases (9.8%) and two false-negative cases (3.9%), reported Subesinghe, who provides regular specialist radiology support for the Leeds Specialist Skin Cancer MDT Centre.
"The retrospective nature of this study and the relatively small cohort coupled with an unequal proportion of examinations performed in stage IV disease are potential limitations as they may positively skew the results and contribute to the high estimation of the clinical impact of FDG PET/CT," he conceded. "Reporting of the FDG PET/CT examination with a lack of blinding to the result of the prior [contrast-enhanced] CT was likely to influence the positive clinical impact of FDG PET/CT, but this methodology is reflective of real life, day-to-day practice."
In addition, the median delay between the CT scan and subsequent FDG PET/CT examination of 32 days may have been sufficient time for new sites of disease to develop in the intervening period and become apparent on FDG PET/CT, thereby falsely resulting in a major clinical impact by detection of additional sites of disease, according to the authors.
They think prospective trials in stage-specific cohorts of patients, in addition to further cost-effective analyses, are now required to develop consensus opinion on the utilization of FDG PET/CT in MM.
Editor's Note: The image on our home page is of a human melanoma cell line growing in tissue culture.