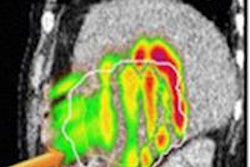
Radiotherapy of the lungs and heart have saved many patients' lives, but because radiation also harms the heart, the organ should generally be evaluated following cancer treatment, especially for higher-risk groups of patients, according to a Belgian-authored article in the European Heart Journal.
Cardiac toxicity from cancer treatment is serious enough to require routine evaluation and imaging studies to reduce the risk of morbidity and mortality, noted researchers from the Heart Valve Clinic at the University of Liège Hospital and two dozen other centers worldwide. The review paper looks at the evidence for radiation-induced heart disease (RIHD) and reviews the imaging protocols best suited for post-therapeutic assessment.
"Although modern radiotherapy techniques are likely to reduce the prevalence and severity of RIHD, the incidence of RIHD is expected to increase in cancer survivors who have received old radiotherapy regimens," lead author Dr. Patrizio Lancellotti and colleagues wrote. "Improved knowledge of the prevalence of RIHD will help the medical community to better evaluate and inform patients of the risk of RIHD after chest radiotherapy" (Eur Heart J, August 2013, Vol. 14:8, pp. 721-740).
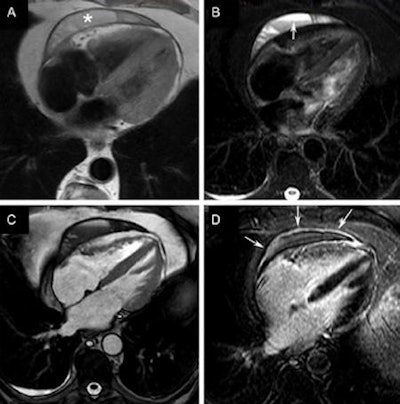 Cardiac CT of the pericardium: normal pericardium (A), thickened pericardium (B), pericardial effusion and hyperenhanced pericardial layers (C), and pericardial calcification (D). All images courtesy of the European Association of Cardiovascular Imaging of the European Society of Cardiology.
Cardiac CT of the pericardium: normal pericardium (A), thickened pericardium (B), pericardial effusion and hyperenhanced pericardial layers (C), and pericardial calcification (D). All images courtesy of the European Association of Cardiovascular Imaging of the European Society of Cardiology.High-dose radiation exposure of the chest comes mainly from adjuvant radiotherapy after breast surgery, radiotherapy of the lung and for esophageal cancer, and radiotherapy complementing systemic treatment for lymphoma, the authors wrote. The damage in RIHD comes from the total cumulative radiation dose resulting from radiotherapy and exacerbated by adjunctive chemotherapy.
RIHD may not become clinically apparent until years after the exposure, they stated. The disease can manifest in a wide range of deleterious effects on the heart, including pericarditis, coronary artery disease, and myocardial infarction. More than 40% of cancer survivors are more than 10 years past their treatment, giving rise to the risk of developing RIHD, a risk that can be exacerbated by other common risk factors, including cardiovascular disease, obesity, inactivity, smoking, and substance abuse, Lancellotti and colleagues wrote.
Several clinical trials and epidemiologic studies have described RIHD's toll on long-term cancer survivors, and clinicians should be on the lookout for signs of disease in daily practice, the authors wrote. The article reviews the literature and offers guidelines on the use of cardiac imaging for the detection and monitoring of RIHD, they wrote.
According to several studies, an estimated 10% to 20% of patients develop RIHD between five and 10 years after treatment, partially negating the long-term benefits of cancer treatment. Tempering that picture somewhat, most available clinical studies are single-center studies that used old radiotherapy techniques and usually didn't obtain baseline exams, according to the study team.
"It appears that the cumulative dose and its fractioning determine acute and chronic cardiac effects of radiation therapy," they wrote. "In the past, pericarditis used to be the most common side effect in patients receiving traditional radiotherapy for Hodgkin's disease. Dose restriction to 30 Gy with lower daily fraction, different weighting of radiation fields, and blocking of the subcarinal region have been reported to reduce the incidence of pericarditis from 20% to 2.5%." Yet the effects of lower doses are less obvious.
Radiation therapy also increases the risk of cardiotoxic effects of certain chemotherapeutic agents, such as anthracyclines, and the harm seems to depend on the total dose of anthracyclines, the study team wrote. Patient age also matters, with younger patients more at risk. Women irradiated for breast cancer at age 35 or younger have 6.5 times the risk of the general population, and more damage is also seen in younger patients with Hodgkin's lymphoma. Other factors include diabetes, hypertension, obesity, and hypercholesterolemia, but studies have not shown an increase in risk after adjusting for underlying risk factors, Lancellotti and colleagues wrote.
The pathophysiology remains poorly understood, but radiation "might harm virtually all cardiac tissues and the underlying pathophysiological mechanisms may be related to micro- and macrovascular damages," they explained. "Early events in the postradiation cascade are loss of endothelial cells with subsequent inflammatory responses, driving the vascular damage." Microvascular damage is associated with eventual fibrosis and diastolic dysfunction and heart failure. Primary radiation fibrosis results not from the radiation itself but relates to a reparative response of heart tissue to the microvascular injury.
"This is a common pathological feature of late radiation tissue complications," they wrote. As for the pathogenesis of radiation-induced coronary artery disease, it shares common pathways with typical coronary disease drive by genetic and exogenous factors. "As exogenous factors have been shown to result in genomic instability, and as low-dose radiation induces long-lasting genomic instability, a synergistic interaction between radiation-induced effects and pathogenic events unrelated to radiation exposure is highly probable."
The clinical results of these changes include pericarditis, valve disease, myocardial damage, microvascular dysfunction, coronary artery disease, and myocardial ischemia, Lancellotti and colleagues wrote, noting wide variations in latency, exposure patterns, and presentations. Cardiovascular complaints after radiotherapy should be investigated, they wrote, while RIHD's later effects usually become clinically manifest several years after radiation.
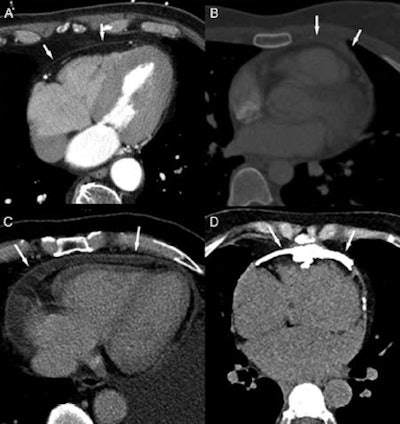 Inflammatory-effusive constrictive pericarditis in a 67-year-old man presenting with increasing complaints of dyspnea. Transthoracic and transesophageal echocardiography were inconclusive to rule out pericardial pathology. Dark-blood, T1-weighted (A), and T2-weighted short-tau inversion recovery (B) fast spin-echo cardiac MR (CMR), CMR (C), and LGE CMR (D). Loculated pericardial effusion (asterisk, A) with several fibrous layers, fluid-fluid level (arrow, B), several fibrous strands, and thickened appearance of the pericardial layers strongly enhancing the following administration of gadolinium contrast agent (arrows, D). The compression of the right ventricular free wall is well visible on CMR (C). Real-time CMR (additional movie) shows inspiratory septal inversion with an increased total respiratory septal shift confirming constrictive component. Pericardiectomy was performed showing chronically inflamed and fibrotically.
Inflammatory-effusive constrictive pericarditis in a 67-year-old man presenting with increasing complaints of dyspnea. Transthoracic and transesophageal echocardiography were inconclusive to rule out pericardial pathology. Dark-blood, T1-weighted (A), and T2-weighted short-tau inversion recovery (B) fast spin-echo cardiac MR (CMR), CMR (C), and LGE CMR (D). Loculated pericardial effusion (asterisk, A) with several fibrous layers, fluid-fluid level (arrow, B), several fibrous strands, and thickened appearance of the pericardial layers strongly enhancing the following administration of gadolinium contrast agent (arrows, D). The compression of the right ventricular free wall is well visible on CMR (C). Real-time CMR (additional movie) shows inspiratory septal inversion with an increased total respiratory septal shift confirming constrictive component. Pericardiectomy was performed showing chronically inflamed and fibrotically.Imaging for RIHD
Echocardiography is central for evaluating the morphology and function of the heart, and results from the symptomatic patient or exam findings. Other modalities, including cardiac CT angiography (CCTA), cardiac MRI (CMR), and nuclear cardiology (SPECT), are used to confirm and evaluate the extent of RIHD, and their clinical utility depends on pathological features.
"For instance, the role of nuclear cardiology for assessing pericardial structures, myocardial fibrosis, or valvular heart disease associated with RIHD is limited by its suboptimal spatial resolution," they wrote. "Conversely, the sensitivity of cardiac CT to detect localized pericardial effusion and pericardial thickening and the accuracy of CMR in characterization of myocardial edema, inflammation, and fibrosis are superior to echocardiography."
Echocardiography
Echo is used to detect structural abnormalities, measure left ventricular (LV) performance, and evaluate valve disease severity. Clinical indications dictate the choice of M-mode, Doppler, 2D or 3D transthoracic or transesophageal, contrast, or stress echocardiography.
Suboptimal visualization of the endocardial border is a common limitation of 2D/3D echocardiography, and is common in patients with obesity, respiratory disease, thoracic deformity, or previous open-chest cardiac surgery, Lancellotti and colleagues wrote. Contrast can help improve endocardial border definition to levels compatible with CMR.
Speckle-tracking echocardiography is accurate for strain quantification. For valve analysis, transthoracic Doppler echo is recommended as the first-line test, while transesophageal echocardiography can be used when transthoracic echocardiography is nondiagnostic; 3D echo is amenable to patients with complex valve lesions, they wrote.
Cardiac MRI
MRI can visualize many RIHD-related pathologies, including epicardial coronary artery stenosis, microvasculature on myocardial perfusion, and ventricular function and viability, the authors noted. Stress myocardial perfusion can identify reversible myocardial ischemia, and CMR is the gold standard for myocardial assessment, and superior to SPECT for detecting hemodynamically significant stenosis. For myocardial edema in the setting of acute myocarditis, T2-weighted fast spin-echo imaging, using a short-tau inversion-recovery (STIR) sequence (triple inversion recovery), depicts increased free water in areas of high signal intensity.
Bright-blood cine CMR using balanced steady-state free precession (SSFP) gradient-echo sequences, provides dynamic information to quantify ventricular volumes, function, and mass; assess regional myocardial function; and visualize valvular heart disease. Myocardial deformation patterns can be evaluated using CMR tagging techniques. Another important CMR technique is velocity-encoded or phase-contrast cine CMR to measure the degree of dephasing caused by through-plane motion of protons. However, CMR suffers from limited availability in many centers and requires the use of contrast to detect coronary artery calcium.
Cardiac CT
CT excels in the assessment of coronary arteries, its ability to evaluate coronary calcium without contrast, and high sensitivity and specificity for ruling out coronary artery disease. And obstructive CAD is likely rare without detectable calcium after irradiation.
CT is hampered by its limited ability to evaluate the severity of stenosis, although emerging applications to determine the hemodynamic significance of coronary stenosis, including stress myocardial perfusion CT and fractional flow-reserve CT computer-simulated fractional flow reserves based on CT could alter the picture. Late contrast enhancement at CT can also pick up myocardial scars.
In limited studies of patients after radiation therapy for Hodgkin's disease, CT found advanced coronary calcification and obstructive coronary disease in the young cohort; however, it is unclear if CT can distinguish general atherosclerotic disease from atherosclerosis caused by radiation therapy, the authors wrote. Absent symptoms of coronary disease, there is probably insufficient data to recommend the use of CT after radiation therapy, the authors wrote.
Nuclear cardiology
The prevalence of myocardial perfusion abnormalities among patients undergoing radiotherapy varies widely. But in one study, the incidence of myocardial perfusion abnormalities increased over time from 27% at six months post-treatment to 42% at 24 months after radiotherapy, they noted. But in patients with relatively large areas of perfusion defects, repeat scanning three to eight years after radiotherapy showed that perfusion abnormalities persisted (Gayed et al).
Coronary CTA
"From the available data, it is unclear whether CT could distinguish general atherosclerotic CAD from lesions caused by radiation therapy," the group wrote. "In the absence of symptoms of CAD, there is currently insufficient data to recommend a routine use of coronary CT angiography in patients who underwent high-dose radiation therapy."
| Overview of imaging techniques for detection and follow-up of RIHD | |||||
| Echocardiography | CMR | Cardiac CT | Stress echo | SPECT | |
| Pericardial disease | |||||
| Effusion screening and positive diagnosis | +++++ | + | + | - | + |
| Effusion follow-up | ++++ | - | - | - | + |
| Constriction | ++++ | ++++ | ++ | - | + |
| Myocardial disease | |||||
| LV systolic dysfunction | ++++ | ++++ | + | ++++ | ++++ |
| LV dysfunction, follow-up | ++++ | + | - | - | ++ |
| Fibrosis | - | ++++ | + | - | - |
| Valve disease | |||||
| Diagnosis and severity | ++++ | ++ | - | ++ | + |
| Follow-up | ++++ | - | - | ++ | + |
| Coronary disease | |||||
| Diagnosis | + | ++++ | ++ | ++++ | + |
| Follow-up | + | + | - | ++++ | + |
The medical literature to date argues in favor of a comprehensive long-term follow-up to develop potential strategies to reduce the risk of RIHD development, the study team wrote. "As the epidemiological studies do not give clues on the important mechanisms underlying RIHD, it is difficult to design preventive strategies. Alteration in radiotherapy field or targeted radiation, with avoidance and/or shielding of the heart, remains one of the most important interventions to prevent RIHD."
The highest-risk patients include those of younger age, with cardiovascular risk factors or pre-existing cardiovascular diseases, exposure to high doses of radiation, anterior or left-chest irradiation location, and absence of shielding, the authors noted. The prevalence of abnormalities increases markedly over time from five to 20 years, "making a strong argument for screening because they are often clinically unrecognized," they wrote. However, the best imaging methods to use in each situation remain unclear.