Robotic technology developer Hansen Medical and Philips Healthcare have strengthened their collaboration on robotic systems for endovascular interventions by securing certified compatibility between their products.
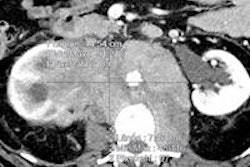
Hansen Medical's Magellan robotic system and Philips' Allura interventional x-ray systems have been shown to be compatible according to article 12 of medical device directive 93/42/EEC, according to the two companies.
Philips began collaborating with Hansen Medical on the development of Magellan in 2010. Hansen received the CE Mark for the system in 2011 and 510(k) clearance from the U.S. Food and Drug Administration (FDA) in 2012, the firms said.