The Contrast Media Safety Committee of the European Society of Urogenital Radiology (ESUR) has updated its guidelines on nephrogenic systemic fibrosis (NSF), which includes the point of caution that patients with a glomerular filtration rate (GFR) below 30 mL/min/1.73 m2 have an increased risk of developing the disease.
Symptoms of NSF include skin lesions, severely disabled impairment of daily activities, and even increased mortality; therefore, it's important to stay on top of NSF guidelines to ensure patient safety.
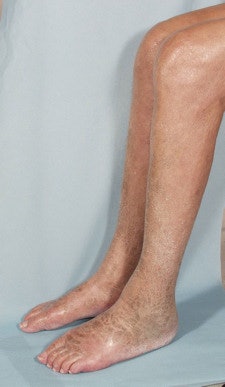
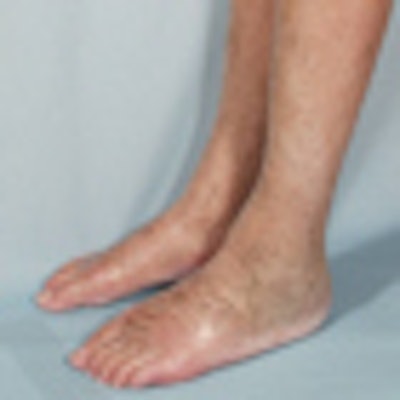
 Skin lesions due to NSF. The legs demonstrate typical skin lesions due to NSF in the lower extremities. All images courtesy of Dr. Henrik Thomsen.
Skin lesions due to NSF. The legs demonstrate typical skin lesions due to NSF in the lower extremities. All images courtesy of Dr. Henrik Thomsen.The ESUR committee produced guidelines on NSF in 2007, but since then more data have emerged, leading the committee to update its guidelines. It also considered the potential long-term problems from retention of small amounts of free gadolinium in the body after procedures enhanced with gadolinium-based contrast media (European Radiology, 6 August 2012).
The PubMed database was systematically reviewed, and more than 656 papers were screened by the committee. The strength of the recommendation and the level of evidence for different prophylactic strategies for NSF were weighted and graded according to predefined scales, according to committee chairman Dr. Henrik Thomsen, the director and a professor in the department of diagnostic sciences at the University of Copenhagen in Denmark.
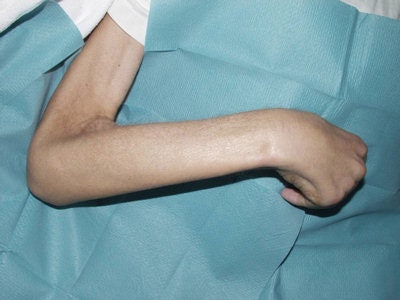 This patient suffered from universal NSF a few days before he died. The arm is fixed, and he looks starved due to the gastrointestinal fibrosis.
This patient suffered from universal NSF a few days before he died. The arm is fixed, and he looks starved due to the gastrointestinal fibrosis.This version of the guidelines has only minor revisions, and the key features remain the need to identify patients with impaired renal function, as well as restrictions placed on giving such patients gadolinium-based contrast media, which are most stringent with the lowest-stability (highest-risk) agents, Thomsen and colleagues wrote.
The committee's recommendations:
The contrast agents with the highest risk of NSF (gadodiamide, gadopentetate dimeglumine, and gadoversetamide) are contraindicated in chronic kidney disease 4 and 5 (GFR less than 30 mL/min/1.73 m2) and for patients on dialysis and those with acute renal insufficiency.
The contrast agents with the highest risk of NSF are contraindicated in neonates and pregnant women.
The contrast agents with the highest risk of NSF should be used with caution in patients with chronic kidney disease 3 (GFR 30-60 mL/min/1.73 m2) with at least seven days between injections.
The contrast agents with the highest risk of NSF should be used with caution in children younger than age 1.
Serum creatinine (eGFR) and clinical assessment of the patient are mandatory before contrast medium administration.
The contrast agents with the highest risk of NSF should never be given in doses greater than 0.1 mmol/kg in any patient.
Contrast agents with intermediate risk of NSF (gadobenate dimeglumine, gadofosvest trisodium, gadoxetate disodium), and contrast agents with lowest risk of NSF (gadobutrol, gadoterate meglumine, and gadoteridol) should be used with caution in patients with chronic kidney disease 4 and 5 (GFR less than 30 mL/min/1.73 m2), including patients on dialysis, with at least seven days between two injections.
Contrast agents with intermediate and low risk of NSF can be used in pregnant women to give essential diagnostic information.
In lactating women, the decision about whether to stop breast-feeding and discard the breast milk for 24 hours after contrast administration should be made by the woman after discussion with the doctor.
Serum creatinine (eGFR) measurement before administration of contrast agents with intermediate and low risk of NSF is not mandatory. Renal function assessment by questionnaire is sufficient.
"An unfortunate result of anxieties about NSF has been that enhancement during MRI may be avoided inappropriately and important disease overlooked," Thomsen and colleagues wrote. "In a patient with mild or moderate renal impairment, the risk of NSF from an MR examination enhanced with one of the most stable gadolinium-based agents is likely to be less than the risk of nephrotoxicity from a CT examination enhanced with an iodine-based agent."



















