Radiographers must be aware that patients with severe renal dysfunction are at higher risk of nephrogenic systemic fibrosis (NSF), and they must check a patient's estimated glomerular filtration rate prior to administration of gadolinium-based contrast agents and ensure that practice and protocols are current and safe, a researcher from Glasgow, U.K., has underlined.
Published guidelines or advice may change as a result of further research -- radiographers must review guidance from bodies such as the Medicines and Healthcare products Regulatory Agency (MHRA) and need to be aware of local policy, according to Sharon Stewart, from the department of psychology and allied health sciences, Glasgow Caledonian University, U.K. Evidence-based practice (EBP) can help to ensure radiographers are practicing safely and effectively.
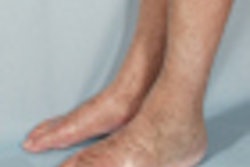
 Nephrogenic systemic fibrosis was first reported in 2000 as a dermopathy of unknown etiology. Image courtesy of Uremic Frost/Kidney International.
Nephrogenic systemic fibrosis was first reported in 2000 as a dermopathy of unknown etiology. Image courtesy of Uremic Frost/Kidney International.
"The emergence of NSF provides an excellent example of why radiographers are required to use EBP," she noted at the 2012 U.K. Radiological Congress (UKRC) in Manchester. "EBP can be used as a tool to ensure safe administration of gadolinium contrast agents for patients with chronic kidney disease."
NSF was first reported in 2000 as a "new" dermopathy of unknown etiology, observed only in patients with chronic kidney disease; it is an incapacitating, potentially fatal disease that primarily affects the skin, and is now known to be a systemic disorder affecting other structures such as muscle and internal organs, according to Stewart. A link between NSF sufferers and gadolinium-based contrast agents used in MRI was suggested in 2006, and the U.S. Food and Drug Administration (FDA) published warnings for health professionals, but the guidelines for administering gadolinium remained confusing and complex, and changed almost daily, she added.
EBP involves integrating the best available evidence with clinical expertise to deliver safe and effective diagnosis, care, and treatment. It is a key part of clinical effectiveness, defined as the right person doing the right thing in the right way at the right time in the right place with the right result, she explained. EBP means reviewing your own practice and questioning if it is safe, effective, and based on evidence.
In the U.K., radiographers administer gadolinium-based contrast agents under Patient Group Direction legislation that identifies radiographers as appropriate professionals for this task, but requires them to keep aware of any changes to recommendations by continuing professional development. Radiographers must keep up to date with current practice, and therefore literature searches are a core skill in modern radiography curricula.
"In NSF, the sheer number (of articles) makes it impossible to review the data, and whilst it includes relevant research papers and reports, it also comprises news items, nonpeer-reviewed items and adverts for law firms. Using appropriate keywords and databases and use of Boolean terms can narrow the search to a more manageable and relevant dataset," she pointed out.
Among the questions that still need to be considered are: Which contrast agents are safe? What is the causal link between NSF and gadolinium contrast agents? Is the chelate structure significant? Is dose relevant? What are the patient risk factors, and at what level of renal dysfunction is it safe to administer agents? Is glomerular filtration rate (GFR) more accurate than serum creatinine to evaluate dysfunction? Are patients with normal kidney function at risk? Does dialysis help postadministration?
Stewart noted recent guidelines are clear on certain points; for instance, the European Society of Urogenital Radiology (European Radiology, 4 August 2012), MHRA, and FDA agree that patients with severe renal impairment (GFR < 30 mL/min) should not be given Omniscan or Magnevist, and they advise caution in patients with moderate renal impairment (GFR 30-59 mL/min). No cases of NSF have been reported in patients with GFR of greater than 60 mL/min, and no new cases of NSF have been reported since these guidelines have been implemented.
"The answers to some other questions have changed and evolved in the last five years and remain in flux. For example, initially dialysis patients were required to have prompt dialysis postcontrast. This is now optional and remains a matter of debate," she concluded.