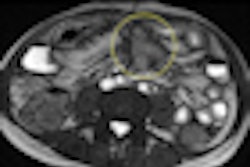
Nordion said that an additional phase III clinical trial for its TheraSphere yttrium-90 (Y-90) glass microsphere treatment for liver cancer will be based primarily in Europe.
The study, called YES-P, will evaluate and compare the safety and efficacy of TheraSphere vs. sorafenib in hepatocellular carcinoma patients with portal vein thrombosis, according to Nordion. Additional locations will also be identified globally for the study, which is targeting enrollment of about 350 patients at approximately 24 sites, Nordion said.
The trial will employ a two-armed design, with patients receiving Y-90 radioembolization treatment with TheraSphere in one arm and sorafenib in the other arm.