When I started training in radiology at London's Hammersmith Hospital in 1981, one of the hot topics was the introduction of new nonionic contrast media. At the Hammersmith, Dr. David Allison was performing a trial of traditional ionic contrast media compared with the new nonionic contrast media in visceral angiography. It was a no-brainer really because the patients had significant pain with traditional ionic agents.
 Dr. Torsten Almén is a pioneer in the development of modern nonionic contrast media. All images courtesy of Dr. Adrian Thomas.
Dr. Torsten Almén is a pioneer in the development of modern nonionic contrast media. All images courtesy of Dr. Adrian Thomas.
The Swedish radiologist Dr. Torsten Almén (b. 1931) made the connection between pain experienced at angiography and osmolarity. He grew up on the most southern coast of Sweden, and recalled a holiday as a boy in Bohuslän on the west coast of Sweden. He found swimming in the water uncomfortable because as soon as he opened his eyes they started to hurt. The salty water at Bohuslän made his eyes sore, whereas the brackish water around Ystad did not cause discomfort. He reasoned that "a plasma-isotonic aqueous solution of contrast medium molecules might not cause pain, and should therefore be created!"
Almén's ideas were rejected by several pharmaceutical manufacturers, but Dr. Hugo Holtermann, research director of pharmaceutical company Nyegaard, encouraged his team to attempt synthesis of some of Almén's theoretical molecules. It is remarkable that less than six months were to elapse between the first meeting of Almén and the Nyegaard research group in June 1968 and the production of the first compound. A consultant reviewer of Almén's 1969 paper stated: "The general principles of Dr. Almén's proposal is probably sound. The implementation of it is probably impractical. He seems to be unaware that the ionic nature of the iodinated compounds is an essential property for their solubility in water -- so part of his proposal, i.e., using nonionic hydrophilic compounds, may be invalid."
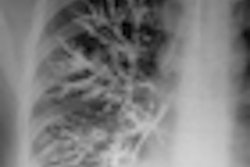
 Above: Metrizamide (Amipaque) (Reproduced from Thomas and Grainger, 1999). Below: Metrizamide (Amipaque), Nyegaard & Co, Oslo, Norway.
Above: Metrizamide (Amipaque) (Reproduced from Thomas and Grainger, 1999). Below: Metrizamide (Amipaque), Nyegaard & Co, Oslo, Norway.

In November 1969, after biological and pharmacological testing, compound 16 (called "Sweet Sixteen") was shown to be the most promising, and it was marketed as Amipaque, which was the first low-osmolar contrast medium. Amipaque was based on the glucose amide of Isopaque (metrizoate), leading to its generic name metrizamide. Because it contained the glucose radical, metrizamide could not be autoclaved; its production was relatively complex, being presented as a freeze-dried powder with a diluent. It was, however, a major toxicological improvement on all pre-existing water-soluble myelographic and vascular agents, and in the late 1970s, it became the internationally recognized agent for myelography enabling water-soluble myelography to replace oil (Myodil, Pantopaque) myelography. Although it had an advantageous intravascular profile, metrizamide was generally regarded as being unsuitable for vascular studies.
Deservedly, Almén was presented with the Antoine Béclère Prize at the 1989 World Congress of Radiology in Paris.
In the mid 1970s, metrizamide was replaced by the second-generation low-osmolar contrast media iohexol (Omnipaque) and iopamidol (Niopam), which are easier to synthesize and therefore less expensive. They did not contain the glucose radical and could therefore be autoclaved and were stable in solution. Further developments have resulted in the development of nonionic dimers (iotrolan and iodixanol).
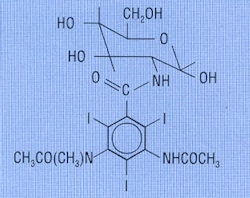
 Selectans: Nonionic Selectan neutral (upper) and ionic Uroselectan (lower) (Reproduced from Thomas and Grainger, 1999).
Selectans: Nonionic Selectan neutral (upper) and ionic Uroselectan (lower) (Reproduced from Thomas and Grainger, 1999).
The original intravascular contrast media were based on the work of Arthur Binz and Curt Räth from Berlin who, in 1925 and 1926, synthesized organic iodine and arsenic compounds based on the pyridine ring in an attempt to produce an agent to treat infections. The pyridine ring is six-pointed and made up of five carbon atoms and one nitrogen atom. One group of iodinated pyridine compounds was found to be selectively excreted by the liver and kidney, and was therefore called the Selectans.
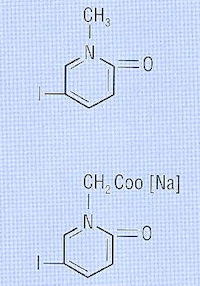
In 1928, Dr. Moses Swick (1900-1985) from the U.S. was awarded the Libman Scholarship and went to work with Dr. Leopold Lichtwitz in Hamburg, Germany. Swick had some success in the treatment of human biliary coccal infections with the Selectans, and since these contained iodine, it occurred to Swick that they might be of value in visualizing the renal tract by x-rays. The initial studies were encouraging, and Swick moved to Berlin to work with Dr. Alexander von Lichtenberg. The first successful human intravenous urograms were produced with (nonionic) N methyl-5-iodo-2 pyridone (Selectan neutral), but Swick preferred the less toxic, more soluble salt 5-iodo-2-pyridone-N-acetate sodium (Uroselectan). This new compounds produced excellent quality intravenous urograms with relatively little toxicity.
 Intravenous urogram (1929) by Dr. Moses Swick (Reproduced from Thomas and Grainger, 1999).
Intravenous urogram (1929) by Dr. Moses Swick (Reproduced from Thomas and Grainger, 1999).
Swick and von Lichtenberg presented the work at the Ninth Congress of the German Urological Society in September 1929. Within two years, Binz and Räth developed two further modifications of the pyridine ring -- diodrast (Diodone) and neo-ipax (Uroselectan B, Iodoxyl) -- each molecule containing two iodine atoms. These compounds were successful and were the standard intravascular and urologic contrast media for the next 20 years.
In the forefront of these developments has been the synthesis of safer, more effective, more physiological, lower osmolar water-soluble iodinated contrast media and methods for their delivery. This has led the way for intravascular interventional therapy, which has successfully challenged and in several areas has replaced conventional surgery. However, we should remember how good the early contrast media were, and that the first successful agent was nonionic.
Dr. Adrian Thomas is chairman of the International Society for the History of Radiology and honorary librarian at the British Institute of Radiology.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.
Further reading
Grainger RG, Thomas AMK. History of intravascular iodinated contrast media. In: Dawson P, Cosgrove D, Allison, DJ, eds. Textbook of Contrast Media. Oxford, U.K.: Isis Medical Media; 1999.
Thomas AMK, Banerjee AK, Busch U. Classic Papers in Modern Diagnostic Radiology. Berlin, NY: Springer Verlag; 2005.