
A new dual-energy CT electronic cleansing technique thoroughly outperformed an advanced single-source CT cleansing method in virtual colonoscopy (also known as CT colonography or CTC) in a recent study. The technique eliminated many common artifacts that have plagued investigational electronic cleansing techniques.
The impressive results -- although preliminary and limited thus far to studies in plastic and animal phantoms -- show the technique's potential to breathe new life into the quest for a minimal-prep VC screening method that maintains high sensitivity for colonic polyps while rendering the process easier for patients to endure and radiologists to read.
Skipping the bowel prep
In clinical practice, both conventional and virtual colonoscopy generally require a cathartic bowel prep, which discourages many patients from undergoing routine colorectal cancer screening.
For radiologists, cathartic bowel preparations in virtual colonoscopy produce superior image quality that makes studies faster and easier to interpret accurately.
But cathartic bowel cleansing "is very uncomfortable, and makes it hard for the patient to accept CTC screening," said Wenli Cai, PhD, from Massachusetts General Hospital (MGH) in Boston, who presented the research at the 2010 Computer Assisted Radiology and Surgery (CARS) meeting in Geneva.
One solution is to offer so-called electronic cleansing, which uses thresholding and gradient analysis to distinguish colonic mucosa from tagged stool and fluid, which are then removed from the images.
But the cleansing algorithms -- many created by MGH researchers -- create problems of their own in the form of severe cleansing artifacts that impair the diagnostic utility of the CTC images.
The low quality of traditional electronic cleansing "is caused by pseudo-soft-tissue structures, which are basically caused by partial-volume effects between the air and the air-tagging boundaries," and a thin soft-tissue structure just one or two voxels thick that is sandwiched between the air-filled lumen and the tagged region, Cai explained.
Unfortunately, when these pseudo-soft-tissue structures are removed by the image-processing technique, parts of the colonic mucosa disappear with it. Other artifacts are caused by inhomogeneous tagging, he said.
The study aimed to develop an electronic cleansing method for noncathartic dual-energy CTC (DE-CTC) to minimize the artifacts. The researchers tested their method in a plastic colon phantom study and in phantoms made of pig colons to compare a previously developed structural analysis electronic cleansing (SA-EC) method with DE-CTC.
Dual-energy CT scanners acquire the same anatomic volume in two energies (80 kVp and 140 kVp), enabling the differentiation of materials by decomposing attenuation coefficients, Cai said.
Plastic phantom
Cai and his colleagues from the institution's 3D laboratory, June-Goo Lee; Bob Liu, PhD; and Hiro Yoshida, PhD, built an anthropomorphic colon phantom and filled it with "simulated tagged materials," consisting of psyllium husk mixed with ground cereal and oral iodinated contrast agent (1 L of 20 mg/mL concentration of iohexol, Omnipaque, GE Healthcare, Chalfont St. Giles, U.K.).
The phantom was scanned on a dual-source CT scanner (Somatom Definition, Siemens Healthcare, Malvern, PA) at two tube energies: 80 kVp and 140 kVp.
"We also fused the images to simulate [single-source] 120-kVp images," Cai said. All the images were then subjected to a previously validated SA-EC scheme.
An expectation maximization algorithm within the image classifier was used to estimate the material coefficient vector of each voxel and the optimal model parameters, yielding a maximum probability for the expected mixtures of low- and high-attenuation materials, Cai explained.
A regional material decomposition method was then used to segment the phantom model, creating a cleansing mask of removed materials and a preservation mask representing colonic mucosa for the DE-CTC images, Cai said. A Markov random field was added to smooth out image noise artifacts.
In the resulting masks, air, tagging, and the air-tagging boundary were subtracted from the image while everything else, representing the colonic mucosa, was preserved.
Finally, the tagging phantom was registered with the native phantom, and the registered images were cleansed using DE-CTC.
DE-CT was qualitatively indistinguishable from the native (empty) colon phantom and far superior to the synthesized single-source electronic cleansing process in the example of a supine image, even in the detail images, Cai said.
 Above, DE-CT provides high-quality electronic cleansing that is nearly as clean as the native phantom, and far superior to the synthesized single-source electronic cleansing process. Bottom, detail view shows how DE-CT process successfully removed thin-layer tagging and inhomogeneous contrast tagging while leaving the mucosa intact. All images courtesy of Wenli Cai, PhD, and Hiro Yoshida, PhD.
Above, DE-CT provides high-quality electronic cleansing that is nearly as clean as the native phantom, and far superior to the synthesized single-source electronic cleansing process. Bottom, detail view shows how DE-CT process successfully removed thin-layer tagging and inhomogeneous contrast tagging while leaving the mucosa intact. All images courtesy of Wenli Cai, PhD, and Hiro Yoshida, PhD.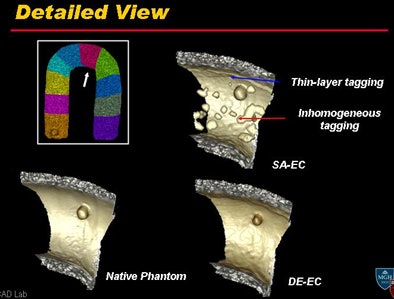
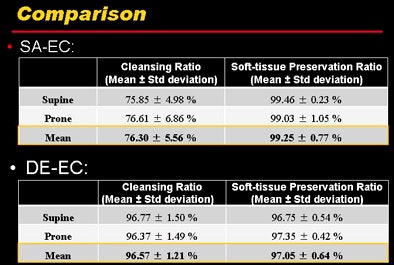 Quantitative results show that both cleansing and soft-tissue preservation were far superior in DE-CT compared to (synthesized) single-source CT electronic cleansing.
Quantitative results show that both cleansing and soft-tissue preservation were far superior in DE-CT compared to (synthesized) single-source CT electronic cleansing."Clearly, the soft-tissue material is still preserved in the images using the method," he said.
Pig colon phantom test
For further study, the group created three additional phantoms out of pig colons about 1-m long each, including 12 polyps ranging in size from 6-13 mm that were randomly placed in the mucosa. The colons were filled with the same cereal/contrast mixture and scanned with DE-CT using the same parameters.
For evaluation, each pig colon was divided into 10 segments and examined using a segmental unblinding technique.
|
Having trouble viewing this clip? Click here to view full-size clip or to change format. |
| Video clip demonstrating the effect of dual-energy electronic cleansing on a pig phantom. Video courtesy of Wenli Cai, PhD. |
The dual-energy electronic cleansing (DE-EC) results in the pig phantoms revealed an average per-polyp sensitivity and specificity of 92% and 94% for the detection of all polyps 6 mm and larger, respectively, and 100% sensitivity and specificity for polyps 8 mm and larger.
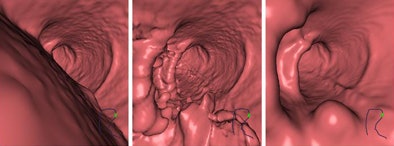 3D endoscopic views of a polyp in a pig colon after (left) no electronic cleansing, i.e., original CTC data; (middle) structure-analysis electronic cleansing applied to single-energy CTC data; and (right) dual-energy electronic cleansing.
3D endoscopic views of a polyp in a pig colon after (left) no electronic cleansing, i.e., original CTC data; (middle) structure-analysis electronic cleansing applied to single-energy CTC data; and (right) dual-energy electronic cleansing.In this small study, the DE-CT method was shown to reduce both principal kinds of electronic-cleansing artifacts: pseudo-soft-tissue structures and inhomogeneous tagging, Cai said.
The study showed that dual-energy electronic cleansing "has the potential to substantially improve the quality of EC" in noncathartic CTC, Cai said. "The method could potentially be useful for [detecting] submerged flat lesions."
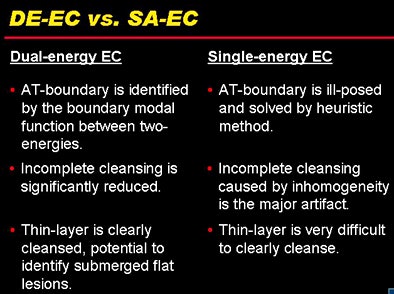 DE-CT cleansing holds several advantages over single-source structured analysis cleansing.
DE-CT cleansing holds several advantages over single-source structured analysis cleansing.The group's next step will be applying the technique in clinical studies, Cai said. Although the radiation dose depends on user-defined scan parameters, it is not significantly higher than generated in a single-source scan, Cai said in response to a question from the session moderator.
By Eric Barnes
AuntMinnie.com staff writer
July 21, 2010
Related Reading
Requiring prone and supine CAD marks boosts VC results, July 14, 2010
VC CAD: Projection-based features reduce false positives, March 10, 2010
VC CAD application does well alone and as reader aid, February 15, 2010
New VC CAD algorithm reveals submucosal colon cancers, February 4, 2010
Sophisticated electronic bowel cleansing boosts polyp detection, August 3, 2009
Copyright © 2010 AuntMinnie.com

















