LONDON (Reuters), Mar 2 - New imaging technology suggests an experimental drug for Alzheimer's reduces clumps of plaque in the brain by around 25%, giving potential new life to a medicine that disappointed in clinical tests two years ago.
Bapineuzumab -- being developed by Pfizer Inc, Irish drugmaker Elan Corp, and Johnson & Johnson -- could be the first drug to treat the underlying cause of the degenerative brain disease.
In July 2008, however, the drug failed to meet its main goal in a midstage trial and caused brain swelling at higher doses. The new study, which only involved 28 patients, is a modest boost.
"It demonstrated that the drug has an effect on the pathological hallmark of Alzheimer's disease," lead researcher Juha Rinne from Finland's University of Turku told Reuters.
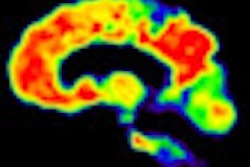
Rinne and colleagues used a novel imaging substance that sticks to areas of the brain where there is a lot of the beta amyloid plaque seen in Alzheimer's.
After 78 weeks, they found that patients given bapineuzumab had about a 25% reduction in plaque compared with those on placebo, or dummy pills. The effect was similar with three different doses of the drug, they reported in the journal Lancet Neurology.
The treatment was generally well tolerated, although two patients on the highest dose had short-term brain swelling. The drug's developers have since dropped the top dose from large ongoing later-stage trials.
Commenting on the results, Sam Gandy from New York's Mount Sinai School of Medicine said it was too early to say effective disease-modifying drugs were at hand, but the ability to measure plaque in living subjects was "something of a breakthrough."
Experts are divided on the root cause of Alzheimer's and hence the best way to tackle it.
Several drugs are now approved for the disease, including Namenda (memantine) and Aricept (donepezil). None of those available drugs stop the disease, but only treat the symptoms.
Most of those in the pipeline, like bapineuzumab, have focused on removing clumps of amyloid plaques, which are thought to stop brain cells from functioning properly. But a rival school blames toxic tangles caused by an abnormal build-up of another protein, tau.
Rinne's imaging study was funded by Elan and Wyeth, which is now part of Pfizer.
By Ben Hirschler
Source: Lancet Neurology, online March 1, 2010.
Last Updated: 2010-03-01 12:20:13 -0400 (Reuters Health)
Copyright © 2010 Reuters Limited. All rights reserved. Republication or redistribution of Reuters content, including by framing or similar means, is expressly prohibited without the prior written consent of Reuters. Reuters shall not be liable for any errors or delays in the content, or for any actions taken in reliance thereon. Reuters and the Reuters sphere logo are registered trademarks and trademarks of the Reuters group of companies around the world.