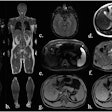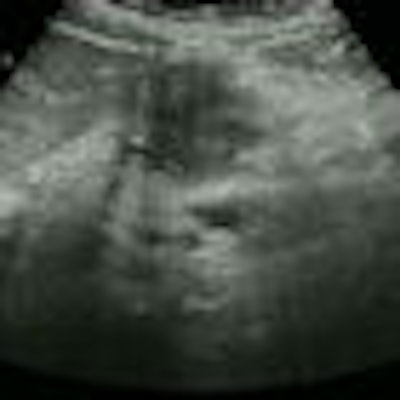
SAN FRANCISCO - Ultrasound detected small pancreatic cancers even CT and MRI couldn't visualize in a novel Dutch screening program aimed at high-risk patients with a family history of the disease. The researchers hope to transform their successful pilot study into an international multicenter trial aimed at finding pancreatic cancer in high-risk patients while it's still resectable.
Pancreatic cancer is one of the largest cancer killers in both the U.S. and Europe, with a dismal overall five-year survival rate of about 3%, said Dr. Jan-Werner Poley from Erasmus University Rotterdam in the Netherlands.
"This is at least partly due to the fact that only a small minority [10% to 20%] of patients are amenable to surgical resection at diagnosis, and even with resection, five-year survival is only 10% to 20%," Poley said on Friday at the 2009 Gastrointestinal Cancers Symposium.
Of course, the low incidence of the disease precludes screening in the general population, but it's a different story in patients at high risk of disease. Of the approximately 1,700 new cases of pancreatic cancer reported each year in the Netherlands, about 10% to 15% have a hereditary component.
Hereditary pancreatic cancer patients consist mainly of two broad types, with familial pancreatic cancers (FPCs) comprising the largest group, and a much smaller group of inherited tumor syndromes and diseases, Poley explained.
FPCs "have no underlying gene defect, but presumed disease-causing mutations appear to inherit in an autosomal dominant pattern," he said. Inherited syndromes, on the other hand, are associated with a gene defect and comprise about 20% of hereditary pancreatic cancer cases. Disease prevalence and survival vary widely depending on the particular syndrome and the family that inherits it, with a cumulative lifetime risk in these patients ranging from 3% to 40%.
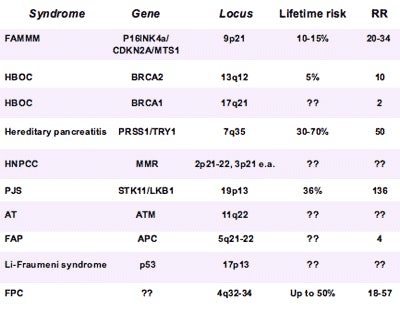 |
| Inherited gene mutations and syndromes with increased risk for pancreatic cancer include approximately 80% of familial pancreatic cancers and about 20% of gene mutations. Risks that remain poorly understood are represented by question marks. Data courtesy of Dr. Jan-Werner Poley. |
For high-risk populations such as these, screening makes sense for several reasons, Poley said.
"Hereditary cases tend to develop cancer at a considerably younger age compared with controls, and the odds for a cure are minimal once pancreatic cancer has become symptomatic," he said. "Moreover, in recent year it's become clear that precancerous precursor lesions exist."
Also, the ability to visualize precursor lesions such as intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasia (IPMN), and small solid lesions means it's possible to perform curative resection in some cases before cancer develops.
And if one is determined to screen, endoscopic ultrasonography (EUS) is the way to do it, Poley said. Recently, Canto and colleagues (Clinical Gastroenterology and Hepatology, 2005, 2006) showed that EUS has the highest sensitivity of all imaging modalities for detecting pancreatic cancer, especially in hereditary populations. EUS can safely guide fine-needle aspiration and has virtually no complications, Poley said.
On the other hand, EUS is invasive, usually requires conscious sedation, and is highly operator-dependent. But the sonographers at Erasmus University Medical Center in Rotterdam, along with their colleagues at the Dutch Cancer Center and Academic Medical Centre in Amsterdam, had performed EUS thousands of times, Poley said.
Their pilot study aimed to demonstrate the feasibility of screening high-risk patients with EUS at the centers.
In all, they scanned 43 asymptomatic patients (18 men, 25 women; mean age, 51) who were known mutation carriers, were at 50% or greater risk of pancreatic cancer, or had a lifetime risk of 10% or greater. The three experienced sonographers used radial or linear instruments according to their personal preference. If abnormalities were detected at EUS, the patient was followed up with CT and/or MRI.
The subjects' diverse genetic backgrounds included:
- FPC (n = 21)
- Familial atypical multiple mole melanoma pancreatic cancer (FAMMM-PC) (n = 13)
- Hereditary pancreatitis (n = 3)
- Peutz-Jeghers syndrome (n = 2)
- BRCA1/2 (n = 3/2)
- p53 mutation (n = 1)
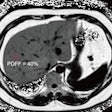
Among the 43 patients, the researchers found three asymptomatic mass lesions in the body or tail of the pancreas (12 mm, 27 mm, and 50 mm). Two of these were from patients from genetically proven FAMMM syndrome families, and one patient was from a BRCA2 family with a cluster of pancreatic carcinoma.
The smallest lesion (12 mm) was not visible on either CT or MRI; the two smallest lesions (12 mm, 27 mm) were resected.
"All of the cases were moderately differentiated adenocarcinoma, and unfortunately the patients with the two largest lesions (27 mm, 50 mm) already had nodal disease," Poley said.
"Unfortunately, the patient with the 27-mm adenocarcinoma died approximately 15 months after surgery due to local recurrence, and the other two patients are currently undergoing chemotherapy due to liver metastases," he said.
|
Having trouble viewing this clip? Click here to view full-size clip or to change format. |
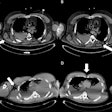
| At EUS screening, 27-mm adenocarcinoma can be seen in the tail of the pancreas near the splenic artery and vein. All videos courtesy of Dr. Jan-Werner Poley. |
|
Having trouble viewing this clip? Click here to view full-size clip or to change format. |
| At EUS screening, solid cystic lesion (IPMN) is seen in the tail of the pancreas; patient has a BRCA1 mutation. |
There were also seven patients (3 FAMMM, 3 FPC, 1 BRCA1) with branch-type IPMN-appearing lesions ranging in size from 6 mm to 15 mm with no sign of malignancy. Incidental findings included a left adrenal mass (p53 mutation patient), a nonmalignant 25-mm adenoma, and a 15-mm lesion in the left liver lobe, diagnosed as a hepatic adenoma after fine-needle aspiration and MRI.
"We've shown that EUS is able to detect early precursor lesions in hereditary pancreatic cancer," Poley said. The detection of three pancreatic cancers in 6.5% of the study group was an "unprecedented high yield of one-time screening with EUS in high-risk patients," he said. The study also revealed eight precursor lesions in 17.4% of the subjects and two incidental findings in 4.4% of subjects.
The study raises a number of questions, for example, about the proper role of MRI, which is less invasive but probably less sensitive than EUS, he said.
"How should we follow up, and what is the natural history of precursor lesions in patients with hereditary pancreatic cancer?" Poley asked. "What's the impact on survival and what's the psychological impact of screening."
Poley said he believes that EUS-based screening should be done as a research protocol. The Netherlands team has begun a comparative study with MRI and is working with U.S. researchers in the hope of establishing an international consortium.
By Eric Barnes
AuntMinnie.com staff writer
January 19, 2009
DNA variations may predict outcome in pancreas cancer, January 15, 2009
Perfusion CT predicts treatment response for pancreatic cancer, January 2, 2009
Radiation therapy prior to pancreatic cancer resection improves survival, December 9, 2008
Ultrasound of the pancreas: Cystic and non-focal pathology, February 13, 2004
Focal solid lesions of the pancreas, October 14, 2003
Copyright © 2009 AuntMinnie.com