A CT-based prognostic radiomic signature for non-small cell lung cancer (NSCLC) can be ported for use with cone-beam CT (CBCT) images, reports a new study by researchers from the Netherlands. The approach could potentially allow treatments to be monitored and adjusted in their early stages to afford better outcomes for patients (Radiotherapy & Oncology, June 2017, Vol. 123:3, pp. 363-369).
Radiomics -- the analysis of quantitative features extracted from medical images -- has the potential to shed light on a patient's response to treatment and their likelihood of developing side effects. In most patient treatments, however, medical images -- whether CT, MRI, or PET -- are taken only at a limited number of points in time, if at all beyond the treatment planning stage. This hampers their effectiveness in aiding treatment adaptation.
In the treatment of NSCLC, however, it is standard procedure to routinely take 3D CBCT scans for patient setup and position verification. If radiomics methods could be applied to these images, then it would be possible to better monitor early tumor responses to radiation therapy and adapt treatment plans accordingly. This would be of particular benefit for lung cancer patients, who often face poor prognoses, and whose outcomes would likely greatly benefit from more personalized treatment.
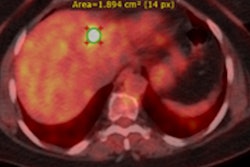
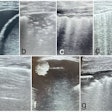
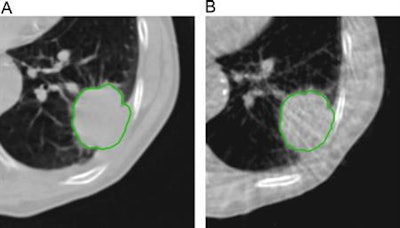 Example of planning CT and CBCT images. A: Treatment planning CT scan. B: kV cone-beam CT scan of the same patient prior to the first treatment fraction. The gross tumor volume is indicated in green. © 2017 van Timmeren JE, Leijenaar RTH, van Elmpt W, et al. (Radiother Oncol, June 2017, Vol. 123:3, pp. 363-369).
Example of planning CT and CBCT images. A: Treatment planning CT scan. B: kV cone-beam CT scan of the same patient prior to the first treatment fraction. The gross tumor volume is indicated in green. © 2017 van Timmeren JE, Leijenaar RTH, van Elmpt W, et al. (Radiother Oncol, June 2017, Vol. 123:3, pp. 363-369).To explore the potential interchangeability of CT and CBCT radiomic features, Janna van Timmeren from the MAASTRO clinic and colleagues looked at 288 patients from three clinics who had previously been treated for NSCLC. The researchers compared treatment planning CT scans for each patient with the corresponding CBCT image taken prior to the first radiotherapy fraction.
Following this and a two-step correction procedure, they evaluated a prognostic radiomic signature developed for CT images of NSCLC patients -- capable of sorting into low- and high-risk categories for overall survival -- for its portability across to the CBCT scans.
Common features
The researchers found that a subset (13.3%) of radiomic features was interchangeable between CT and CBCT images via a simple linear regression. Filtered radiomic features were not interchangeable, van Timmeren explained, as they require a margin around the region of interest that cannot always be accommodated by CBCT images, which often have a limited longitudinal field-of-view.
More important, the team found that the four features that made up the CT-based radiomic signature they tested also provided prognostic value when derived from the CBCT images -- meaning that analysis of the latter could potentially be used to predict treatment outcomes.
"Since CBCT images are usually routinely acquired weekly or daily for lung cancer patients, it is an ideal modality for monitoring tumor changes over the course of treatment, which can potentially be used for early treatment adaptation," van Timmeren said.
"The authors carefully evaluated the potential of CBCT radiomics after CBCT intensity correction and confirmed the interchangeability of radiomic features extracted from both CBCT and conventional CT," commented Tianye Niu, a medical physicist from Zhejiang University who was not involved in this study. With CBCT image faithfully monitoring the day-to-day variation of tumors during treatment, originally CT-based radiomic signatures can be applied to CBCT images for survival prediction in non-small cell lung cancer patients, he said.
"Timmeren and colleagues thus open a promising way to early treatment adaptation using radiomics based on CBCT imaging over time," Niu concluded.
Having demonstrated the potential of CBCT-based radiomics, the researchers now plan to explore potential delta radiomics applications to inform early treatment adaption.
Ian Randall is a science writer based in New Zealand.