Hybrid imaging systems provide powerful tools for the detection and surveillance of disease, but manufacturers have been slow to provide the software that can help physicians recognize and interpret artifacts, a leading pioneer in the field believes.
Faster adaptation of attenuation correction technology to hybrid imaging machines could help sort out the distorting effects of implants, patient motion, or other factors that can skew images, according to Thomas Beyer, PhD, professor of physics of medical imaging and deputy head of the Centre for Medical Physics and Biomedical Engineering at the Medical University of Vienna.
"What is noticeable is that the different algorithms that are available in standalone machines, like CT or MRI, are not immediately available for correcting the same artifacts in the combined imaging exams," said Beyer, who works on the development of PET/CT and PET/MRI systems.
Software to correct for metal artifacts was available for CT scanners, but not for PET/CT machines, which made their commercial debut in 2001, until a few years later, he noted. "This is something that is annoying. Industry typically says it's to do with software release, but quite frankly it can't be a barrier."
ECR delegates will learn today about how regular technology updates, along with more collaboration amongst physicians, physicists and technologists, can help to avoid errors in hybrid imaging. They will also find out about the challenges, but also how to identify and interpret artifacts that persist, even as technology becomes more sophisticated.
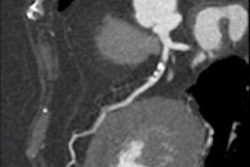
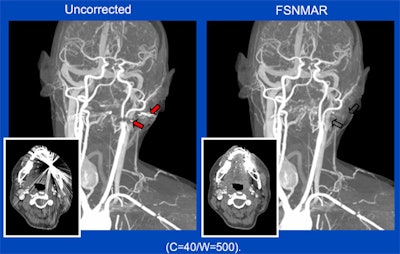 Metal artifacts in CT can be caused by very dense (x-ray opaque) objects, such as metal implants or highly concentrated intravenous contrast agents. In the shadow of the metal objects, virtually no primary x-rays are received by the detector and a conventional reconstruction will, thus, produce significant artifacts (left). A new algorithm -- frequency-split normalized metal artifact reduction (FSNMAR), or iterative metal artifact reduction (iMAR) -- helps to fill in the missing data with consistent values, thereby outperforming other known metal artifact reduction techniques. Images courtesy of Dr. Thomas Beyer.
Metal artifacts in CT can be caused by very dense (x-ray opaque) objects, such as metal implants or highly concentrated intravenous contrast agents. In the shadow of the metal objects, virtually no primary x-rays are received by the detector and a conventional reconstruction will, thus, produce significant artifacts (left). A new algorithm -- frequency-split normalized metal artifact reduction (FSNMAR), or iterative metal artifact reduction (iMAR) -- helps to fill in the missing data with consistent values, thereby outperforming other known metal artifact reduction techniques. Images courtesy of Dr. Thomas Beyer.For instance, hybrid imaging systems give doctors increasingly comprehensive molecular and anatomical information, but the combination can also trigger distortion. As Beyer explains, CT transmission information has been used for attenuation and scatter correction of tracers in PET scans, but while beneficial for improving the accuracy of diagnosis, it actually is the one that causes most of the artifacts.
Attenuation correction has also proved to be a challenge for PET/MRI, which is seen as a possible breakthrough in oncology for its ability to simultaneously provide molecular, functional, and anatomical information. Patient motion, leading to misalignment of data between MRI and PET, has proved to be a particular concern, according to Dr. Harald Quick, director of the University of Duisburg's Erwin L. Hahn Institute for MR Imaging in Essen, Germany.
In addition, obesity and metal prostheses also can constrain MR field-of-view. Hip implants, for example, are known to create large-volume artifacts and signal voids in MR images that could affect diagnostic assessments of surrounding tissues, he explained.
"MR-based attenuation correction is still a relatively new concept that is under constant evaluation and further development," he added. "While it works robustly for most cases with the already established standard methods and MR imaging sequences, new methods are being developed to further improve the robustness and the accuracy of MR-based attenuation correction."
Researchers are focusing on several improvements to address image accuracy. These include the use of continuous attenuation values for multiple tissue classes, detection of bone tissue, assignment of different attenuation correction values for lung tissue of differing density, motion correction measures, correction of truncated areas, and reducing metal artifacts.
"This all might sound as if we are still a long way from home," Quick said. "However, to provide a number, we are fighting for the last 10% of improvement when we compare MR-based attenuation correction in PET/MR to the established method of CT-based attenuation correction in PET/CT."
His recommendation to PET/MR image readers is to always examine the nonattenuation correction and attenuation correction PET images, along with the MR-based attenuation correction images, to help identify artifacts. In addition, physicians need to be cautious when reporting specific active lesions or tumors, he said. Also, the true activity of PET-visible lesions close to artifacts in the MR-based attenuation correction images might be underestimated or overrated by a few percent.
The prognosis for correcting the loss of imaging energy through obstructions, scatting, and other causes seems to be good.
"With PET/MRI, we currently see the implementation of bone tissue in the head region by using ultrashort echo time sequences, the addition of bones in the body by using atlas-based methods, the correction of truncation artifacts using new sequences that are able to extend the MR imaging field-of-view (HUGE), the correction of motion artifacts in thorax and liver imaging, and finally, the reduction of metal artifacts around implants using appropriate metal artifact reduction sequences," Quick stated.
Meanwhile, Beyer thinks the next big steps in technology can help compensate for motion, while new algorithms will address the disruption created by large implants. He sees today's ECR discussions as one way to drive home the need for training and collaboration between disciplines (nuclear medicine and radiology, as well as physicists and clinical technologists) to pick up and interpret artifacts in PET/CT and the emerging field of PET/MRI. He also encourages practitioners to know the guidelines published for hybrid imaging.
"We need to prepare people to open their mind if they are interested in hybrid imaging," he said. "In other words, neither a radiologist nor a nuclear medicine physician as the primary stakeholders could launch into a hybrid imaging operation without recognizing the know-how and strengths of the other expert."
This does not only pertain to doctors but also to technologists and physicists, Beyer continued. "To us it is very important that we drive a session where we don't look at this from an ivory tower, but we really give practical examples and try to relay complex causes of artifacts in simple words."
Originally published in ECR Today on 2 March 2016.
Copyright © 2016 European Society of Radiology